-
PDF
- Split View
-
Views
-
Cite
Cite
Michael D Ezekowitz, Charles V Pollack, Jonathan L Halperin, Richard D England, Sandra VanPelt Nguyen, Judith Spahr, Maria Sudworth, Nilo B Cater, Andrei Breazna, Jonas Oldgren, Paulus Kirchhof, Apixaban compared to heparin/vitamin K antagonist in patients with atrial fibrillation scheduled for cardioversion: the EMANATE trial, European Heart Journal, Volume 39, Issue 32, 21 August 2018, Pages 2959–2971, https://doi.org/10.1093/eurheartj/ehy148
Close - Share Icon Share
Abstract
The primary objective was to compare apixaban to heparin/vitamin K antagonist (VKA) in patients with atrial fibrillation (AF) and ≤48 h anticoagulation prior to randomization undergoing cardioversion.
One thousand five hundred patients were randomized. The apixaban dose of 5 mg b.i.d. was reduced to 2.5 mg b.i.d. in patients with two of the following: age ≥ 80 years, weight ≤ 60 kg, or serum creatinine ≥ 133 µmol/L. To expedite cardioversion, at the discretion of the investigator, imaging and/or a loading dose of 10 mg (down-titrated to 5 mg) was allowed. The endpoints for efficacy were stroke, systemic embolism (SE), and death. The endpoints for safety were major bleeding and clinically relevant non-major (CRNM) bleeding.
There were 1038 active and 300 spontaneous cardioversions; 162 patients were not cardioverted. Imaging was performed in 855 patients, and 342 received a loading dose of apixaban. Comparing apixaban to heparin/VKA in the full analysis set, there were 0/753 vs. 6/747 strokes [relative risk (RR) 0; 95% confidence interval (95% CI) 0–0.64; nominal P = 0.015], no SE, and 2 vs. 1 deaths (RR 1.98; 95% CI 0.19–54.00; nominal P > 0.999). In the safety population, there were 3/735 vs. 6/721 major (RR 0.49; 95% CI 0.10–2.07; nominal P = 0.338) and 11 vs. 13 CRNM bleeding events (RR 0.83; 95% CI 0.34–1.89; nominal P = 0.685). On imaging, 60/61 with thrombi continued randomized treatment; all (61) were without outcome events.
Rates of strokes, systemic emboli, deaths, and bleeds were low for both apixaban and heparin/VKA treated AF patients undergoing cardioversion.
NCT02100228
Introduction
Managing patients with newly diagnosed atrial fibrillation (AF) involves controlling rate, finding reversible causes, preventing stroke, and often restoring sinus rhythm by electrical or pharmacological cardioversion.1 , 2 The risk of periprocedural thromboembolism in patients undergoing cardioversion may exceed 5% when anticoagulation is inadequate.3 , 4 Anticoagulation with a vitamin K antagonist (VKA) for at least 3 weeks prior to cardioversion, during the procedure, and for a minimum of 4 weeks afterwards to allow restoration of atrial mechanical function reduces the risk to less than 1%.5–7 In post hoc analyses of cardioversions in the trials that led to regulatory approval of the thrombin inhibitor dabigatran and the factor Xa inhibitors rivaroxaban, apixaban, and edoxaban, event rates were low.8–11 A limitation of translating these data to clinical practice was the prolonged period of anticoagulation preceding cardioversion.
To accelerate cardioversion in clinical practice, a strategy of imaging by computerized tomography (CT) or transoesophageal echocardiography (TOE) may be used to exclude left atrial thrombus.1 , 2 Randomized open-label trials comparing rivaroxaban and edoxaban to heparin/VKA in patients undergoing elective cardioversion found stroke and major bleeding rates at or below 1% in patients randomized to the factor Xa inhibitors.12 , 13 Apixaban, an orally active, reversible, direct inhibitor of factor Xa, reduced stroke and systemic embolism (SE), major bleeding, and death in patients with AF compared to warfarin in a large randomized double-blind trial, but has not been evaluated prospectively in patients undergoing cardioversion.14
Objectives and endpoints
The primary objective of Eliquis evaluated in acute cardioversion coMpared to usuAl treatmeNts for AnticoagulaTion in subjEcts with atrial fibrillation (EMANATE) was to assess the occurrence of stroke, SE, and all-cause death (efficacy endpoints), and major and clinically relevant non-major (CRNM) bleeding events (safety endpoints) in patients with AF undergoing cardioversion during anticoagulation with either apixaban or a conventional heparin/VKA regimen and ≤48 h of anticoagulation prior to randomization. This study was also designed to assess the safety of a loading dose of apixaban and the utility of image guidance to expedite cardioversion.15
Methods
EMANATE was a multinational, randomized, active-controlled, open-labelled study in patients with recently diagnosed AF scheduled for cardioversion. The design has been described (Figure 1).15 Briefly, patients with electrocardiographically confirmed AF and ≤48 h of prior anticoagulation were randomized 1:1 to apixaban or usual care with parenteral heparin and/or an oral VKA. Randomization used a centralized interactive voice-response system.
Study design. *The protocol encouraged a single loading dose of apixaban either 10 mg or 5 mg and image-guided at the discretion of the investigator, to allow more rapid transition to cardioversion. *Dosage was reduced to 2.5 mg b.i.d. or a single loading dose of 5 mg when two of the following were present: age ≥80 years, weight ≤60 kg, or serum creatinine ≥1.5 mg/dL (133 μmol/L). **Imaging guidance (transoesophageal echocardiography or computer tomography) was at the discretion of the investigator. b.i.d., twice daily; VKA, vitamin K antagonist.
For patients randomized to usual care with heparin and a VKA, the target international normalized ratio (INR) was 2.0–3.0. Heparin was to be continued until the INR was 2 or higher. Patients randomized to apixaban received 5 mg twice daily (b.i.d.), with a dose reduction to 2.5 mg b.i.d. in those with two of the following: age ≥80 years, weight ≤60 kg, or serum creatinine ≥1.5 mg/dL (133 µmol/L), mirroring approved dosing for stroke prevention in patients with non-valvular AF.16 Cardioversion was allowed after five doses of apixaban, when steady state blood levels are expected to be achieved. To expedite cardioversion, at the discretion of the investigator, in patients randomized to apixaban, a single 10 mg dose of apixaban could be administered to achieve exposure at 2 h similar to steady state. When the maintenance dose was down-titrated, the loading dose was reduced to 5 mg. Cardioversion could be performed 2 h after administration of the loading dose. For patients in the usual care arm, similar timing was allowed after administration of heparin.
Local investigators determined the timing and type of cardioversion (pharmacological, electrical, or both). Patients could be randomized and cardioverted on the same day. Cardioversion could be performed up to a maximum of 90 days after randomization (Figure 1). All cardioverted patients continued anticoagulation and were to be followed for 30 ± 7 days to complete the study.15 Follow-up for those not cardioverted was completed at 90 ± 7 days (Figure 1). At the end of the study, or upon early withdrawal, the patient’s subsequent management and treatment was the decision of the treating physician. Transition procedures followed the apixaban label.16
Patients could withdraw from the study at any time of their own volition, at the discretion of the investigator or sponsor for safety or behavioural reasons, or due to the inability of the patient to comply with the required schedule of visits or procedures. Patients had the right to withdraw and refuse further contact, but every effort was made to ensure follow-up for outcomes relevant to the study objectives.
Imaging
Transoesophageal echocardiographic or CT imaging was performed at the direction of the investigator and interpreted locally. When left atrial thrombus was identified, investigators were instructed to defer cardioversion, continue the randomized anticoagulant, and repeat imaging after ∼3 weeks before proceeding to cardioversion.
Management of bleeding
In the event of clinically significant bleeding, anticoagulation was interrupted and managed according to local practice with surgical haemostasis, volume repletion, and transfusion of blood products and for patients in the heparin/VKA arm, administration of protamine, supplemental vitamin K or fresh frozen plasma as deemed appropriate by the treating physician.
Outcomes assessment
Efficacy (stroke, SE, all-cause death) was assessed in all randomized patients, defined as the intention-to-treat population. Safety (major bleeds and CRNM bleeds) was assessed in patients receiving at least one dose of study anticoagulant according to their actual treatment received, defined as the safety population.
Acute stroke was defined as a focal neurological deficit of sudden onset, lasting at least 24 h, not due to a non-vascular cause. Strokes were classified as primary ischaemic, haemorrhagic, infarction with haemorrhagic conversion, or of unknown type if no imaging was available.17 , 18 Extracranial SE events were defined by a clinical presentation consistent with acute loss of blood supply to an anatomical site supplied by a single artery, supported by evidence from angiography, surgical specimens, autopsy, or other objective testing. The definition of major bleeding was adapted from the International Society on Thrombosis and Haemostasis19 , 20 as clinically overt bleeding accompanied by one or more of the following: a decrease in haemoglobin of ≥1.24 mmol/L; transfusion of ≥2 units of packed red blood cells; bleeding that occurs in at least one of the following critical sites: intracranial, intraspinal, intraocular, pericardial, intra-articular, intramuscular with compartment syndrome, retroperitoneal; or bleeding that is fatal. The definition of CRNM bleeding was adapted from the International Society on Thrombosis and Haemostasis criteria as overt bleeding that compromised haemodynamics, led to hospitalization, produced subcutaneous haematoma >25 cm2, or 100 cm2 if traumatic, intramuscular haematoma documented by ultrasonography, epistaxis lasting >5 min or leading to intervention.15 , 20
Serious adverse events
Serious adverse events (SAEs) were untoward medical occurrences that were life-threatening or fatal, required inpatient hospitalization or prolonged an existing hospitalization, resulted in persistent or significant disability or incapacity, required intervention to prevent permanent impairment or damage, or based on medical judgement were important medical events that placed a patient in jeopardy and required medical or surgical intervention to prevent one of the aforementioned outcomes.
Sample size determination and statistical analysis
As there was no precedent for prospective evaluation of patients undergoing cardioversion using apixaban, the warfarin-naïve cohort from the ARISTOTLE trial14 was considered most applicable to this study. In ARISTOTLE, the incidence of stroke and SE within 30 days after randomization was 0.3% and the incidence of major bleeding was 0.45%. Adequate power to assess for non-inferiority required 480 endpoints.14 , 15 With follow-up limited to 30 days following cardioversion or 90 days post-randomization, an event rate of 1% would require approximately 48 000 patients, a number far in excess of practicality. EMANATE is a descriptive study without hypothesis testing. There was no formal power calculation regarding the sample size. A sample size of 1500 patients was considered clinically meaningful and achievable and is similar to the sample size used for assessment of another factor Xa inhibitor in patients with AF undergoing cardioversion.12
The efficacy endpoints (stroke, system embolic, all-cause death) were analysed based on full analysis data set defined as all randomized patients under the intent to treat principle. The safety endpoints (major bleeding and CRNM bleeding) were analysed based on safety analysis set defined as all treated patients (randomized patients who received at least 1 dose of study drug), analysed according to actual treatment received. For stroke, SE, all-cause death, major bleeding, and CRNM bleeding, the event rates and their exact 95% confidence intervals (CIs) were presented by treatment group; exact relative risks (RR) between two treatment groups were presented with 95% exact unconditional CI. The nominal P-value from a Fisher’s exact test between two treatment groups was also presented. Time to event was displayed using the Kaplan–Meier curve.
The analysis of time to cardioversion defined as the time from first dose to cardioversion was post hoc. The mean, standard deviation, and 95% CI of the mean were presented by treatment group, and by image guidance status within each treatment group. The comparison of time to cardioversion with and without image guidance in each treatment group and with and without an apixaban loading dose in apixaban was analysed using a t-test. The 95% CI of mean difference for the comparisons were also provided.
Results
The study was conducted between 14 July 2014 and 08 February 2017 at 134 centres in 12 countries. Enrolled patients were required to have ≤48 h of anticoagulation prior to randomization, and 930 (62%) had none. Atrial fibrillation was first detected within 3 months before entry in 1170 (78%; Table 1). Among 1500 randomized patients, 753 were assigned to apixaban and 747 to heparin/VKA (Table 1, Figure 2). Of these, 98% completed follow-up; one patient assigned to heparin/VKA was lost to follow-up 1 day after randomization. Among patients randomized to heparin/VKA the INR was ≥2.0 for 65% of the time beyond the first two weeks of treatment. In the apixaban group, 91% of patients had compliance estimated by pill count of >80%.
Baseline demographics (intention-to-treat population)
| . | Heparin/ VKA . | Apixaban . | Apixaban loading dose 5 mg . | Apixaban loading dose 10 mg . | Spontaneous cardioversiona . | Active cardioversiona . | No cardioversiona . | Electrical cardioversionb . | Pharmacological cardioversiona . | Electrical + pharmacological cardioversiona . |
|---|---|---|---|---|---|---|---|---|---|---|
| Number of patients | 747 | 753 | 11 | 331 | 300 | 1038 | 162 | 925 | 65 | 52 |
| Apixaban : heparin/VKA | 162 : 138 | 519 : 519 | 72 : 90 | 461 : 464 | 35 : 30 | 23 : 29 | ||||
| Age (years), mean ± SD | 64.5 ± 12.8 | 64.7 ± 12.2 | 80.5 ± 7.4 | 63.2 ± 12.2 | 63.3 ± 13.1 | 64.5 ± 12.3 | 67.1 ± 12.1 | 64.9 ± 12.3 | 59 ± 13.2 | 64.9 ± 11.0 |
| Sex, n (%) | ||||||||||
| Male | 497 (66.5) | 505 (67.1) | 7 (63.6) | 208 (62.8) | 184 (61.3) | 713 (68.7) | 105 (64.8) | 635 (68.6) | 45 (69.2) | 35 (67.3) |
| Female | 250 (33.5) | 248 (32.9) | 4 (36.4) | 123 (37.2) | 116 (38.7) | 325 (31.3) | 57 (35.2) | 290 (31.4) | 20 (30.8) | 17 (32.7) |
| Race, n (%) | ||||||||||
| White | 648 (86.7) | 654 (86.9) | 10 (90.9) | 322 (97.3) | 255 (85.0) | 933 (89.9) | 114 (70.4) | 833 (90.1) | 62 (95.4) | 42 (80.8) |
| Black | 20 (2.7) | 21 (2.8) | 1 (9.1) | 6 (1.8) | 11 (3.7) | 20 (1.9) | 10 (6.2) | 20 (2.2) | 0 | 0 |
| Asian | 76 (10.2) | 78 (10.4) | 0 | 3 (0.9) | 33 (11.0) | 83 (8.0) | 38 (23.5) | 70 (7.6) | 3 (4.6) | 10 (19.2) |
| Other | 3 (0.4) | 0 | 0 | 0 | 1 (0.3) | 2 (<0.1) | 0 | 2 (0.2) | 0 | 0 |
| Hypertension, n (%) | ||||||||||
| Yes | 481 (64.4) | 496 (65.9) | 9 (81.8) | 221 (66.8) | 195 (65.0) | 679 (65.4) | 103 (63.6) | 603 (65.2) | 44 (67.7) | 34 (65.4) |
| No | 263 (35.2) | 256 (34.0) | 2 (18.2) | 110 (33.2) | 105 (35.0) | 359 (34.6) | 55 (34.0) | 322 (34.8) | 21 (32.3) | 18 (34.6) |
| Not reported | 3 (0.4) | 1 (0.1) | 0 | 0 | 0 | 0 | 4 (2.5) | 0 | 0 | 0 |
| LVEF <40%, n (%) | ||||||||||
| Yes | 54 (7.2) | 45 (6.0) | 0 | 21 (6.3) | 4 (1.3) | 75 (7.2) | 20 (12.3) | 67 (7.2) | 3 (4.6) | 5 (9.6) |
| No | 690 (92.4) | 703 (93.4) | 10 (90.9) | 309 (93.4) | 296 (98.7) | 961 (92.6) | 136 (84.0) | 857 (92.6) | 61 (93.8) | 47 (90.4) |
| Not reported | 3 (0.4) | 5 (0.7) | 1 (9.1) | 1 (0.3) | 0 | 2 (0.2) | 6 (3.7) | 1 (0.1) | 1 (1.5) | 0 |
| Diabetes, n (%) | ||||||||||
| Yes | 140 (18.7) | 154 (20.5) | 1 (9.1) | 75 (22.7) | 52 (17.3) | 204 (19.6) | 38 (23.5) | 169 (18.3) | 18 (27.7) | 18 (34.6) |
| No | 604 (80.9) | 598 (79.4) | 10 (90.9) | 256 (77.3) | 248 (82.7) | 834 (80.0) | 120 (74.1) | 756 (81.7) | 47 (72.3) | 34 (65.4) |
| Not reported | 3 (0.4) | 1 (0.1) | 0 | 0 | 0 | 0 | 4 (2.5) | 0 | 0 | 0 |
| First documentation of AF, n (%) | ||||||||||
| New onset | 504 (67.5) | 505 (67.1) | 10 (90.9) | 243 (73.4) | 227 (75.7) | 682 (65.7) | 100 (61.7) | 611 (66.1) | 41 (63.1) | 33 (63.5) |
| <3 months | 82 (11.0) | 81 (10.8) | 0 | 22 (6.7) | 22 (7.3) | 118 (11.4) | 23 (14.2) | 112 (12.1) | 3 (4.6) | 4 (7.7) |
| ≥3 months | 157 (21.0) | 165 (21.9) | 1 (9.1) | 66 (19.9) | 51 (17.0) | 236 (22.7) | 35 (21.6) | 200 (21.6) | 21 (32.3) | 15 (28.9) |
| Not reported | 4 (0.5) | 1 (0.1) | 0 | 0 | 0 | 2 (0.2) | 4 (2.5) | 2 (0.2) | 0 | 0 |
| CHA2DS2-VASc Score, mean ± SD | 2.4 ± 1.7 | 2.4 ± 1.7 | 4.4 ± 1.8 | 2.3 ± 1.7 | 2.3 ± 1.6 | 2.4 ± 1.7 | 2.8 ± 1.8 | 2.4 ± 1.7 | 2.1 ± 1.7 | 2.8 ± 2.0 |
| Systolic BP (mmHg), mean ± SD | 129.7 ± 18.2 | 130.2 ± 18.2 | 139.2 + 21.8 | 131.7 ± 18.3 | 128.5 ± 18.1 | 130.2 ± 18.1 | 130.8 ± 19.3 | 130.5 ± 18.1 | 126.5 ± 16.6 | 130.2 ± 19.0 |
| Diastolic BP (mmHg), mean ± SD | 81.7 ± 13.1 | 81.1 ± 12.7 | 83.7 ± 15.8 | 81.0 ± 13.8 | 80.2 ± 12.5 | 81.7 ± 12.7 | 81.7 ± 14.5 | 81.7 ± 12.7 | 80.7 ± 13.4 | 83.4 ± 13.5 |
| Heart rate (beats/min), mean ± SD | 99.2 ± 26.6 | 99.6 ± 25.4 | 101.4 ± 22.8 | 106.1 ± 25.4 | 103.2 ± 28.1 | 98.7 ± 25.2 | 97.0 ± 26.8 | 98.1 ± 25.1 | 105.3 ± 25.1 | 101.1 ± 27.4 |
| Antithrombotic treatment <48 h, n (%) | ||||||||||
| Yes | 289 (38.7) | 287 (38.1) | 7 (63.6) | 173 (52.3) | 94 (31.3) | 431 (41.5) | 51 (31.5) | 374 (40.4) | 33 (50.8) | 25 (48.1) |
| None | 458 (61.3) | 466 (61.9) | 4 (36.4) | 158 (47.7) | 206 (68.7) | 607 (58.5) | 111 (68.5) | 551 (59.6) | 32 (49.2) | 27 (51.9) |
| . | Heparin/ VKA . | Apixaban . | Apixaban loading dose 5 mg . | Apixaban loading dose 10 mg . | Spontaneous cardioversiona . | Active cardioversiona . | No cardioversiona . | Electrical cardioversionb . | Pharmacological cardioversiona . | Electrical + pharmacological cardioversiona . |
|---|---|---|---|---|---|---|---|---|---|---|
| Number of patients | 747 | 753 | 11 | 331 | 300 | 1038 | 162 | 925 | 65 | 52 |
| Apixaban : heparin/VKA | 162 : 138 | 519 : 519 | 72 : 90 | 461 : 464 | 35 : 30 | 23 : 29 | ||||
| Age (years), mean ± SD | 64.5 ± 12.8 | 64.7 ± 12.2 | 80.5 ± 7.4 | 63.2 ± 12.2 | 63.3 ± 13.1 | 64.5 ± 12.3 | 67.1 ± 12.1 | 64.9 ± 12.3 | 59 ± 13.2 | 64.9 ± 11.0 |
| Sex, n (%) | ||||||||||
| Male | 497 (66.5) | 505 (67.1) | 7 (63.6) | 208 (62.8) | 184 (61.3) | 713 (68.7) | 105 (64.8) | 635 (68.6) | 45 (69.2) | 35 (67.3) |
| Female | 250 (33.5) | 248 (32.9) | 4 (36.4) | 123 (37.2) | 116 (38.7) | 325 (31.3) | 57 (35.2) | 290 (31.4) | 20 (30.8) | 17 (32.7) |
| Race, n (%) | ||||||||||
| White | 648 (86.7) | 654 (86.9) | 10 (90.9) | 322 (97.3) | 255 (85.0) | 933 (89.9) | 114 (70.4) | 833 (90.1) | 62 (95.4) | 42 (80.8) |
| Black | 20 (2.7) | 21 (2.8) | 1 (9.1) | 6 (1.8) | 11 (3.7) | 20 (1.9) | 10 (6.2) | 20 (2.2) | 0 | 0 |
| Asian | 76 (10.2) | 78 (10.4) | 0 | 3 (0.9) | 33 (11.0) | 83 (8.0) | 38 (23.5) | 70 (7.6) | 3 (4.6) | 10 (19.2) |
| Other | 3 (0.4) | 0 | 0 | 0 | 1 (0.3) | 2 (<0.1) | 0 | 2 (0.2) | 0 | 0 |
| Hypertension, n (%) | ||||||||||
| Yes | 481 (64.4) | 496 (65.9) | 9 (81.8) | 221 (66.8) | 195 (65.0) | 679 (65.4) | 103 (63.6) | 603 (65.2) | 44 (67.7) | 34 (65.4) |
| No | 263 (35.2) | 256 (34.0) | 2 (18.2) | 110 (33.2) | 105 (35.0) | 359 (34.6) | 55 (34.0) | 322 (34.8) | 21 (32.3) | 18 (34.6) |
| Not reported | 3 (0.4) | 1 (0.1) | 0 | 0 | 0 | 0 | 4 (2.5) | 0 | 0 | 0 |
| LVEF <40%, n (%) | ||||||||||
| Yes | 54 (7.2) | 45 (6.0) | 0 | 21 (6.3) | 4 (1.3) | 75 (7.2) | 20 (12.3) | 67 (7.2) | 3 (4.6) | 5 (9.6) |
| No | 690 (92.4) | 703 (93.4) | 10 (90.9) | 309 (93.4) | 296 (98.7) | 961 (92.6) | 136 (84.0) | 857 (92.6) | 61 (93.8) | 47 (90.4) |
| Not reported | 3 (0.4) | 5 (0.7) | 1 (9.1) | 1 (0.3) | 0 | 2 (0.2) | 6 (3.7) | 1 (0.1) | 1 (1.5) | 0 |
| Diabetes, n (%) | ||||||||||
| Yes | 140 (18.7) | 154 (20.5) | 1 (9.1) | 75 (22.7) | 52 (17.3) | 204 (19.6) | 38 (23.5) | 169 (18.3) | 18 (27.7) | 18 (34.6) |
| No | 604 (80.9) | 598 (79.4) | 10 (90.9) | 256 (77.3) | 248 (82.7) | 834 (80.0) | 120 (74.1) | 756 (81.7) | 47 (72.3) | 34 (65.4) |
| Not reported | 3 (0.4) | 1 (0.1) | 0 | 0 | 0 | 0 | 4 (2.5) | 0 | 0 | 0 |
| First documentation of AF, n (%) | ||||||||||
| New onset | 504 (67.5) | 505 (67.1) | 10 (90.9) | 243 (73.4) | 227 (75.7) | 682 (65.7) | 100 (61.7) | 611 (66.1) | 41 (63.1) | 33 (63.5) |
| <3 months | 82 (11.0) | 81 (10.8) | 0 | 22 (6.7) | 22 (7.3) | 118 (11.4) | 23 (14.2) | 112 (12.1) | 3 (4.6) | 4 (7.7) |
| ≥3 months | 157 (21.0) | 165 (21.9) | 1 (9.1) | 66 (19.9) | 51 (17.0) | 236 (22.7) | 35 (21.6) | 200 (21.6) | 21 (32.3) | 15 (28.9) |
| Not reported | 4 (0.5) | 1 (0.1) | 0 | 0 | 0 | 2 (0.2) | 4 (2.5) | 2 (0.2) | 0 | 0 |
| CHA2DS2-VASc Score, mean ± SD | 2.4 ± 1.7 | 2.4 ± 1.7 | 4.4 ± 1.8 | 2.3 ± 1.7 | 2.3 ± 1.6 | 2.4 ± 1.7 | 2.8 ± 1.8 | 2.4 ± 1.7 | 2.1 ± 1.7 | 2.8 ± 2.0 |
| Systolic BP (mmHg), mean ± SD | 129.7 ± 18.2 | 130.2 ± 18.2 | 139.2 + 21.8 | 131.7 ± 18.3 | 128.5 ± 18.1 | 130.2 ± 18.1 | 130.8 ± 19.3 | 130.5 ± 18.1 | 126.5 ± 16.6 | 130.2 ± 19.0 |
| Diastolic BP (mmHg), mean ± SD | 81.7 ± 13.1 | 81.1 ± 12.7 | 83.7 ± 15.8 | 81.0 ± 13.8 | 80.2 ± 12.5 | 81.7 ± 12.7 | 81.7 ± 14.5 | 81.7 ± 12.7 | 80.7 ± 13.4 | 83.4 ± 13.5 |
| Heart rate (beats/min), mean ± SD | 99.2 ± 26.6 | 99.6 ± 25.4 | 101.4 ± 22.8 | 106.1 ± 25.4 | 103.2 ± 28.1 | 98.7 ± 25.2 | 97.0 ± 26.8 | 98.1 ± 25.1 | 105.3 ± 25.1 | 101.1 ± 27.4 |
| Antithrombotic treatment <48 h, n (%) | ||||||||||
| Yes | 289 (38.7) | 287 (38.1) | 7 (63.6) | 173 (52.3) | 94 (31.3) | 431 (41.5) | 51 (31.5) | 374 (40.4) | 33 (50.8) | 25 (48.1) |
| None | 458 (61.3) | 466 (61.9) | 4 (36.4) | 158 (47.7) | 206 (68.7) | 607 (58.5) | 111 (68.5) | 551 (59.6) | 32 (49.2) | 27 (51.9) |
AF, atrial fibrillation; BP, blood pressure; CHA2DS2-VASc, Congestive heart failure, Hypertension, Age ≥75 years (doubled), Diabetes, Prior stroke or TIA or thromboembolism (doubled), Vascular disease, Age 65–74 years, female Sex category; LVEF, left ventricular ejection fraction; SD, standard deviation; VKA, vitamin K antagonist.
Based on first cardioversion.
Four electrical cardioversions were performed on patients who initially spontaneously cardioverted and then reverted back to atrial fibrillation.
Baseline demographics (intention-to-treat population)
| . | Heparin/ VKA . | Apixaban . | Apixaban loading dose 5 mg . | Apixaban loading dose 10 mg . | Spontaneous cardioversiona . | Active cardioversiona . | No cardioversiona . | Electrical cardioversionb . | Pharmacological cardioversiona . | Electrical + pharmacological cardioversiona . |
|---|---|---|---|---|---|---|---|---|---|---|
| Number of patients | 747 | 753 | 11 | 331 | 300 | 1038 | 162 | 925 | 65 | 52 |
| Apixaban : heparin/VKA | 162 : 138 | 519 : 519 | 72 : 90 | 461 : 464 | 35 : 30 | 23 : 29 | ||||
| Age (years), mean ± SD | 64.5 ± 12.8 | 64.7 ± 12.2 | 80.5 ± 7.4 | 63.2 ± 12.2 | 63.3 ± 13.1 | 64.5 ± 12.3 | 67.1 ± 12.1 | 64.9 ± 12.3 | 59 ± 13.2 | 64.9 ± 11.0 |
| Sex, n (%) | ||||||||||
| Male | 497 (66.5) | 505 (67.1) | 7 (63.6) | 208 (62.8) | 184 (61.3) | 713 (68.7) | 105 (64.8) | 635 (68.6) | 45 (69.2) | 35 (67.3) |
| Female | 250 (33.5) | 248 (32.9) | 4 (36.4) | 123 (37.2) | 116 (38.7) | 325 (31.3) | 57 (35.2) | 290 (31.4) | 20 (30.8) | 17 (32.7) |
| Race, n (%) | ||||||||||
| White | 648 (86.7) | 654 (86.9) | 10 (90.9) | 322 (97.3) | 255 (85.0) | 933 (89.9) | 114 (70.4) | 833 (90.1) | 62 (95.4) | 42 (80.8) |
| Black | 20 (2.7) | 21 (2.8) | 1 (9.1) | 6 (1.8) | 11 (3.7) | 20 (1.9) | 10 (6.2) | 20 (2.2) | 0 | 0 |
| Asian | 76 (10.2) | 78 (10.4) | 0 | 3 (0.9) | 33 (11.0) | 83 (8.0) | 38 (23.5) | 70 (7.6) | 3 (4.6) | 10 (19.2) |
| Other | 3 (0.4) | 0 | 0 | 0 | 1 (0.3) | 2 (<0.1) | 0 | 2 (0.2) | 0 | 0 |
| Hypertension, n (%) | ||||||||||
| Yes | 481 (64.4) | 496 (65.9) | 9 (81.8) | 221 (66.8) | 195 (65.0) | 679 (65.4) | 103 (63.6) | 603 (65.2) | 44 (67.7) | 34 (65.4) |
| No | 263 (35.2) | 256 (34.0) | 2 (18.2) | 110 (33.2) | 105 (35.0) | 359 (34.6) | 55 (34.0) | 322 (34.8) | 21 (32.3) | 18 (34.6) |
| Not reported | 3 (0.4) | 1 (0.1) | 0 | 0 | 0 | 0 | 4 (2.5) | 0 | 0 | 0 |
| LVEF <40%, n (%) | ||||||||||
| Yes | 54 (7.2) | 45 (6.0) | 0 | 21 (6.3) | 4 (1.3) | 75 (7.2) | 20 (12.3) | 67 (7.2) | 3 (4.6) | 5 (9.6) |
| No | 690 (92.4) | 703 (93.4) | 10 (90.9) | 309 (93.4) | 296 (98.7) | 961 (92.6) | 136 (84.0) | 857 (92.6) | 61 (93.8) | 47 (90.4) |
| Not reported | 3 (0.4) | 5 (0.7) | 1 (9.1) | 1 (0.3) | 0 | 2 (0.2) | 6 (3.7) | 1 (0.1) | 1 (1.5) | 0 |
| Diabetes, n (%) | ||||||||||
| Yes | 140 (18.7) | 154 (20.5) | 1 (9.1) | 75 (22.7) | 52 (17.3) | 204 (19.6) | 38 (23.5) | 169 (18.3) | 18 (27.7) | 18 (34.6) |
| No | 604 (80.9) | 598 (79.4) | 10 (90.9) | 256 (77.3) | 248 (82.7) | 834 (80.0) | 120 (74.1) | 756 (81.7) | 47 (72.3) | 34 (65.4) |
| Not reported | 3 (0.4) | 1 (0.1) | 0 | 0 | 0 | 0 | 4 (2.5) | 0 | 0 | 0 |
| First documentation of AF, n (%) | ||||||||||
| New onset | 504 (67.5) | 505 (67.1) | 10 (90.9) | 243 (73.4) | 227 (75.7) | 682 (65.7) | 100 (61.7) | 611 (66.1) | 41 (63.1) | 33 (63.5) |
| <3 months | 82 (11.0) | 81 (10.8) | 0 | 22 (6.7) | 22 (7.3) | 118 (11.4) | 23 (14.2) | 112 (12.1) | 3 (4.6) | 4 (7.7) |
| ≥3 months | 157 (21.0) | 165 (21.9) | 1 (9.1) | 66 (19.9) | 51 (17.0) | 236 (22.7) | 35 (21.6) | 200 (21.6) | 21 (32.3) | 15 (28.9) |
| Not reported | 4 (0.5) | 1 (0.1) | 0 | 0 | 0 | 2 (0.2) | 4 (2.5) | 2 (0.2) | 0 | 0 |
| CHA2DS2-VASc Score, mean ± SD | 2.4 ± 1.7 | 2.4 ± 1.7 | 4.4 ± 1.8 | 2.3 ± 1.7 | 2.3 ± 1.6 | 2.4 ± 1.7 | 2.8 ± 1.8 | 2.4 ± 1.7 | 2.1 ± 1.7 | 2.8 ± 2.0 |
| Systolic BP (mmHg), mean ± SD | 129.7 ± 18.2 | 130.2 ± 18.2 | 139.2 + 21.8 | 131.7 ± 18.3 | 128.5 ± 18.1 | 130.2 ± 18.1 | 130.8 ± 19.3 | 130.5 ± 18.1 | 126.5 ± 16.6 | 130.2 ± 19.0 |
| Diastolic BP (mmHg), mean ± SD | 81.7 ± 13.1 | 81.1 ± 12.7 | 83.7 ± 15.8 | 81.0 ± 13.8 | 80.2 ± 12.5 | 81.7 ± 12.7 | 81.7 ± 14.5 | 81.7 ± 12.7 | 80.7 ± 13.4 | 83.4 ± 13.5 |
| Heart rate (beats/min), mean ± SD | 99.2 ± 26.6 | 99.6 ± 25.4 | 101.4 ± 22.8 | 106.1 ± 25.4 | 103.2 ± 28.1 | 98.7 ± 25.2 | 97.0 ± 26.8 | 98.1 ± 25.1 | 105.3 ± 25.1 | 101.1 ± 27.4 |
| Antithrombotic treatment <48 h, n (%) | ||||||||||
| Yes | 289 (38.7) | 287 (38.1) | 7 (63.6) | 173 (52.3) | 94 (31.3) | 431 (41.5) | 51 (31.5) | 374 (40.4) | 33 (50.8) | 25 (48.1) |
| None | 458 (61.3) | 466 (61.9) | 4 (36.4) | 158 (47.7) | 206 (68.7) | 607 (58.5) | 111 (68.5) | 551 (59.6) | 32 (49.2) | 27 (51.9) |
| . | Heparin/ VKA . | Apixaban . | Apixaban loading dose 5 mg . | Apixaban loading dose 10 mg . | Spontaneous cardioversiona . | Active cardioversiona . | No cardioversiona . | Electrical cardioversionb . | Pharmacological cardioversiona . | Electrical + pharmacological cardioversiona . |
|---|---|---|---|---|---|---|---|---|---|---|
| Number of patients | 747 | 753 | 11 | 331 | 300 | 1038 | 162 | 925 | 65 | 52 |
| Apixaban : heparin/VKA | 162 : 138 | 519 : 519 | 72 : 90 | 461 : 464 | 35 : 30 | 23 : 29 | ||||
| Age (years), mean ± SD | 64.5 ± 12.8 | 64.7 ± 12.2 | 80.5 ± 7.4 | 63.2 ± 12.2 | 63.3 ± 13.1 | 64.5 ± 12.3 | 67.1 ± 12.1 | 64.9 ± 12.3 | 59 ± 13.2 | 64.9 ± 11.0 |
| Sex, n (%) | ||||||||||
| Male | 497 (66.5) | 505 (67.1) | 7 (63.6) | 208 (62.8) | 184 (61.3) | 713 (68.7) | 105 (64.8) | 635 (68.6) | 45 (69.2) | 35 (67.3) |
| Female | 250 (33.5) | 248 (32.9) | 4 (36.4) | 123 (37.2) | 116 (38.7) | 325 (31.3) | 57 (35.2) | 290 (31.4) | 20 (30.8) | 17 (32.7) |
| Race, n (%) | ||||||||||
| White | 648 (86.7) | 654 (86.9) | 10 (90.9) | 322 (97.3) | 255 (85.0) | 933 (89.9) | 114 (70.4) | 833 (90.1) | 62 (95.4) | 42 (80.8) |
| Black | 20 (2.7) | 21 (2.8) | 1 (9.1) | 6 (1.8) | 11 (3.7) | 20 (1.9) | 10 (6.2) | 20 (2.2) | 0 | 0 |
| Asian | 76 (10.2) | 78 (10.4) | 0 | 3 (0.9) | 33 (11.0) | 83 (8.0) | 38 (23.5) | 70 (7.6) | 3 (4.6) | 10 (19.2) |
| Other | 3 (0.4) | 0 | 0 | 0 | 1 (0.3) | 2 (<0.1) | 0 | 2 (0.2) | 0 | 0 |
| Hypertension, n (%) | ||||||||||
| Yes | 481 (64.4) | 496 (65.9) | 9 (81.8) | 221 (66.8) | 195 (65.0) | 679 (65.4) | 103 (63.6) | 603 (65.2) | 44 (67.7) | 34 (65.4) |
| No | 263 (35.2) | 256 (34.0) | 2 (18.2) | 110 (33.2) | 105 (35.0) | 359 (34.6) | 55 (34.0) | 322 (34.8) | 21 (32.3) | 18 (34.6) |
| Not reported | 3 (0.4) | 1 (0.1) | 0 | 0 | 0 | 0 | 4 (2.5) | 0 | 0 | 0 |
| LVEF <40%, n (%) | ||||||||||
| Yes | 54 (7.2) | 45 (6.0) | 0 | 21 (6.3) | 4 (1.3) | 75 (7.2) | 20 (12.3) | 67 (7.2) | 3 (4.6) | 5 (9.6) |
| No | 690 (92.4) | 703 (93.4) | 10 (90.9) | 309 (93.4) | 296 (98.7) | 961 (92.6) | 136 (84.0) | 857 (92.6) | 61 (93.8) | 47 (90.4) |
| Not reported | 3 (0.4) | 5 (0.7) | 1 (9.1) | 1 (0.3) | 0 | 2 (0.2) | 6 (3.7) | 1 (0.1) | 1 (1.5) | 0 |
| Diabetes, n (%) | ||||||||||
| Yes | 140 (18.7) | 154 (20.5) | 1 (9.1) | 75 (22.7) | 52 (17.3) | 204 (19.6) | 38 (23.5) | 169 (18.3) | 18 (27.7) | 18 (34.6) |
| No | 604 (80.9) | 598 (79.4) | 10 (90.9) | 256 (77.3) | 248 (82.7) | 834 (80.0) | 120 (74.1) | 756 (81.7) | 47 (72.3) | 34 (65.4) |
| Not reported | 3 (0.4) | 1 (0.1) | 0 | 0 | 0 | 0 | 4 (2.5) | 0 | 0 | 0 |
| First documentation of AF, n (%) | ||||||||||
| New onset | 504 (67.5) | 505 (67.1) | 10 (90.9) | 243 (73.4) | 227 (75.7) | 682 (65.7) | 100 (61.7) | 611 (66.1) | 41 (63.1) | 33 (63.5) |
| <3 months | 82 (11.0) | 81 (10.8) | 0 | 22 (6.7) | 22 (7.3) | 118 (11.4) | 23 (14.2) | 112 (12.1) | 3 (4.6) | 4 (7.7) |
| ≥3 months | 157 (21.0) | 165 (21.9) | 1 (9.1) | 66 (19.9) | 51 (17.0) | 236 (22.7) | 35 (21.6) | 200 (21.6) | 21 (32.3) | 15 (28.9) |
| Not reported | 4 (0.5) | 1 (0.1) | 0 | 0 | 0 | 2 (0.2) | 4 (2.5) | 2 (0.2) | 0 | 0 |
| CHA2DS2-VASc Score, mean ± SD | 2.4 ± 1.7 | 2.4 ± 1.7 | 4.4 ± 1.8 | 2.3 ± 1.7 | 2.3 ± 1.6 | 2.4 ± 1.7 | 2.8 ± 1.8 | 2.4 ± 1.7 | 2.1 ± 1.7 | 2.8 ± 2.0 |
| Systolic BP (mmHg), mean ± SD | 129.7 ± 18.2 | 130.2 ± 18.2 | 139.2 + 21.8 | 131.7 ± 18.3 | 128.5 ± 18.1 | 130.2 ± 18.1 | 130.8 ± 19.3 | 130.5 ± 18.1 | 126.5 ± 16.6 | 130.2 ± 19.0 |
| Diastolic BP (mmHg), mean ± SD | 81.7 ± 13.1 | 81.1 ± 12.7 | 83.7 ± 15.8 | 81.0 ± 13.8 | 80.2 ± 12.5 | 81.7 ± 12.7 | 81.7 ± 14.5 | 81.7 ± 12.7 | 80.7 ± 13.4 | 83.4 ± 13.5 |
| Heart rate (beats/min), mean ± SD | 99.2 ± 26.6 | 99.6 ± 25.4 | 101.4 ± 22.8 | 106.1 ± 25.4 | 103.2 ± 28.1 | 98.7 ± 25.2 | 97.0 ± 26.8 | 98.1 ± 25.1 | 105.3 ± 25.1 | 101.1 ± 27.4 |
| Antithrombotic treatment <48 h, n (%) | ||||||||||
| Yes | 289 (38.7) | 287 (38.1) | 7 (63.6) | 173 (52.3) | 94 (31.3) | 431 (41.5) | 51 (31.5) | 374 (40.4) | 33 (50.8) | 25 (48.1) |
| None | 458 (61.3) | 466 (61.9) | 4 (36.4) | 158 (47.7) | 206 (68.7) | 607 (58.5) | 111 (68.5) | 551 (59.6) | 32 (49.2) | 27 (51.9) |
AF, atrial fibrillation; BP, blood pressure; CHA2DS2-VASc, Congestive heart failure, Hypertension, Age ≥75 years (doubled), Diabetes, Prior stroke or TIA or thromboembolism (doubled), Vascular disease, Age 65–74 years, female Sex category; LVEF, left ventricular ejection fraction; SD, standard deviation; VKA, vitamin K antagonist.
Based on first cardioversion.
Four electrical cardioversions were performed on patients who initially spontaneously cardioverted and then reverted back to atrial fibrillation.
Three patients died during the study. Twenty-eight patients withdrew and refused further follow-up. Among these, 9 were randomized (3 to apixaban vs. 6 to heparin/VKA), but not dosed or cardioverted, 16 (8 in each group) were followed for a mean of 31.7 days without undergoing cardioversion or developing outcome events, and 3 (2 randomized to apixaban and 1 to heparin/VKA) were actively cardioverted and followed for a mean of 9.7 days after cardioversion without outcome events.
Of all patients randomized, 300 converted to sinus rhythm spontaneously (Tables 1 and 2), and 925 were cardioverted electrically, including 4 who spontaneously cardioverted, then reverted to AF and underwent electrical cardioversion. Sixty-five patients were cardioverted pharmacologically and 52 both pharmacologically and electrically. One patient was actively cardioverted, reverted to AF, then spontaneously reverted to sinus rhythm, and is not included among the 300 spontaneous initial cardioversions (see Table 1).
Time from first dose to active cardioversion (days)
| . | Apixaban . | Heparin/vitamin K antagonist . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| . | Loaded dose . | Non-loaded dose . | |||||||
| . | With image guidance . | Without image guidance . | Overall . | With image guidance . | Without image guidance . | Overall . | With image guidance . | Without image guidance . | Overall . |
| n | 238 | 34 | 272 | 155 | 92 | 247 | 407 | 111 | 518 |
| Mean (95% CI) | 3.3 (2.2–4.4) | 4.1 (0.8–7.4) | 3.4 (2.3–4.4) | 21.7 (18.3–25.0) | 32.5 (29.4–35.5) | 25.7 (23.2–28.2) | 11.5 (9.6–13.5) | 40.7 (35.6–45.7) | 17.8 (15.7–19.9) |
| Median | 1 | 1 | 1 | 15 | 30 | 27 | 2 | 43 | 2 |
| Standard deviation | 8.76 | 9.52 | 8.84 | 21.15 | 14.61 | 19.65 | 19.94 | 26.78 | 24.66 |
| Difference (95% CI) P-valuea | 0.8 (2.4–3.9) P = 0.6214 | 10.8 (5.9–15.7) P < 0.0001 | 29.1 (24.6–33.7) P < 0.0001 | ||||||
| Difference (95% CI) P-valueb | −22.3 (−24.9 to 19.7) P < 0.0001 | ||||||||
| . | Apixaban . | Heparin/vitamin K antagonist . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| . | Loaded dose . | Non-loaded dose . | |||||||
| . | With image guidance . | Without image guidance . | Overall . | With image guidance . | Without image guidance . | Overall . | With image guidance . | Without image guidance . | Overall . |
| n | 238 | 34 | 272 | 155 | 92 | 247 | 407 | 111 | 518 |
| Mean (95% CI) | 3.3 (2.2–4.4) | 4.1 (0.8–7.4) | 3.4 (2.3–4.4) | 21.7 (18.3–25.0) | 32.5 (29.4–35.5) | 25.7 (23.2–28.2) | 11.5 (9.6–13.5) | 40.7 (35.6–45.7) | 17.8 (15.7–19.9) |
| Median | 1 | 1 | 1 | 15 | 30 | 27 | 2 | 43 | 2 |
| Standard deviation | 8.76 | 9.52 | 8.84 | 21.15 | 14.61 | 19.65 | 19.94 | 26.78 | 24.66 |
| Difference (95% CI) P-valuea | 0.8 (2.4–3.9) P = 0.6214 | 10.8 (5.9–15.7) P < 0.0001 | 29.1 (24.6–33.7) P < 0.0001 | ||||||
| Difference (95% CI) P-valueb | −22.3 (−24.9 to 19.7) P < 0.0001 | ||||||||
CI, confidence interval for mean time to cardioversion.
t-test for comparison of time to cardioversion with and without image guidance in each treatment group.
t-test for comparison of time to cardioversion with and without a loading dose (apixaban-treated patients).
Time from first dose to active cardioversion (days)
| . | Apixaban . | Heparin/vitamin K antagonist . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| . | Loaded dose . | Non-loaded dose . | |||||||
| . | With image guidance . | Without image guidance . | Overall . | With image guidance . | Without image guidance . | Overall . | With image guidance . | Without image guidance . | Overall . |
| n | 238 | 34 | 272 | 155 | 92 | 247 | 407 | 111 | 518 |
| Mean (95% CI) | 3.3 (2.2–4.4) | 4.1 (0.8–7.4) | 3.4 (2.3–4.4) | 21.7 (18.3–25.0) | 32.5 (29.4–35.5) | 25.7 (23.2–28.2) | 11.5 (9.6–13.5) | 40.7 (35.6–45.7) | 17.8 (15.7–19.9) |
| Median | 1 | 1 | 1 | 15 | 30 | 27 | 2 | 43 | 2 |
| Standard deviation | 8.76 | 9.52 | 8.84 | 21.15 | 14.61 | 19.65 | 19.94 | 26.78 | 24.66 |
| Difference (95% CI) P-valuea | 0.8 (2.4–3.9) P = 0.6214 | 10.8 (5.9–15.7) P < 0.0001 | 29.1 (24.6–33.7) P < 0.0001 | ||||||
| Difference (95% CI) P-valueb | −22.3 (−24.9 to 19.7) P < 0.0001 | ||||||||
| . | Apixaban . | Heparin/vitamin K antagonist . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| . | Loaded dose . | Non-loaded dose . | |||||||
| . | With image guidance . | Without image guidance . | Overall . | With image guidance . | Without image guidance . | Overall . | With image guidance . | Without image guidance . | Overall . |
| n | 238 | 34 | 272 | 155 | 92 | 247 | 407 | 111 | 518 |
| Mean (95% CI) | 3.3 (2.2–4.4) | 4.1 (0.8–7.4) | 3.4 (2.3–4.4) | 21.7 (18.3–25.0) | 32.5 (29.4–35.5) | 25.7 (23.2–28.2) | 11.5 (9.6–13.5) | 40.7 (35.6–45.7) | 17.8 (15.7–19.9) |
| Median | 1 | 1 | 1 | 15 | 30 | 27 | 2 | 43 | 2 |
| Standard deviation | 8.76 | 9.52 | 8.84 | 21.15 | 14.61 | 19.65 | 19.94 | 26.78 | 24.66 |
| Difference (95% CI) P-valuea | 0.8 (2.4–3.9) P = 0.6214 | 10.8 (5.9–15.7) P < 0.0001 | 29.1 (24.6–33.7) P < 0.0001 | ||||||
| Difference (95% CI) P-valueb | −22.3 (−24.9 to 19.7) P < 0.0001 | ||||||||
CI, confidence interval for mean time to cardioversion.
t-test for comparison of time to cardioversion with and without image guidance in each treatment group.
t-test for comparison of time to cardioversion with and without a loading dose (apixaban-treated patients).
Outcomes
Efficacy outcomes
In the intention-to-treat population, no patients randomized to apixaban developed stroke (0%; 95% CI 0–0.5%), compared to 6 in the heparin/VKA group (0.8%; 95% CI 0.3–1.7%); RR 0; 95% CI 0–0.64; nominal P = 0.015. There were no SE events in either group. There were two deaths in the apixaban arm (0.27%, 95% CI 0.03–0.96%) and 1 in the heparin/VKA arm (0.13%; 95% CI 0–0.74%; RR = 1.98; 95% CI 0.19–54.00; P > 0.9999; Table 4). Most events occurred soon after randomization (Figure 3, Table 4). In the apixaban group, 1 death due to acute alcoholic hepatitis occurred after randomization, but prior to dosing. The second death involved an 81-year-old patient who received a 10 mg loading dose of apixaban, was cardioverted successfully 1 day after randomization, but died 3 weeks later. Death was attributed to perforated colonic diverticulitis, and SE was considered unlikely. The solitary death in the heparin/VKA group occurred suddenly 2 days after randomization and cardioversion (Table 3).
The Kaplan–Meier curve for time to first event for (A) stroke/SE, (B) death, (C) major bleeding, and (D) major/CRNM bleeding from randomization. CRNM, clinically relevant non-major; SE, systemic embolism; VKA, vitamin K antagonist.
Safety outcomes
There were 1456 patients in the safety population, 735 patients in the apixaban group and 721 in the heparin/VKA group. In this cohort, 3 patients developed major bleeding on apixaban (0.41%; 95% CI 0.08–1.2%), vs. 6 on heparin/VKA (0.83%; 95% CI 0.31–1.80%); RR = 0.49; 95% CI 0.10–2.07; P = 0.338. With apixaban, 11 patients developed CRNM bleeding (1.5%; 95% CI 0.75–2.66%) vs. 13 with heparin/VKA (1.8%; 95% CI 0.96–3.06%); RR = 0.83; 95% CI 0.34–1.89; P = 0.685. Most safety events occurred soon after randomization (Figure 3, panels C and D; Tables 4, 5, and 6).
Loading dose of apixaban
Of patients treated with apixaban group, 342 received a loading dose (Figure 2, Table 1); most received 10 mg (n = 331) while 11 received 5 mg. Those given the lower dose were older (mean ± SD; 80.5 ± 7.4 years; Table 1) with lower creatinine clearance (41 ± 13.4 mL/min), and body weight (69 ± 16 kg) compared with those receiving the higher dose (63.2 ± 12.2 years; P < 0.0001); creatinine clearance (91.7 ± 52.1 mL/min; P = 0.0039); and weight (90 ± 21 kg; P = 0.0018). No stroke or SE events occurred among those given the loading dose, but there was 1 death, 1 major bleeding, and 4 CRNM bleeding events (Tables 3, 4, 5, and 6).
Efficacy and safety outcomes
| Efficacy outcomes (intention-to-treat populationa; N = 1500) . | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Apixaban (n = 753) . | Apixaban loading dose (n = 342) . | Heparin/VKA (n = 747) . | |||||||||||||
| . | Total (n = 753) . | Active CV (n = 519) . | Spontaneous CV (n = 162) . | Imaged (n = 418) . | No CV (n = 72) . | Total (n = 342) . | Active CV (n = 272) . | Spontaneous CV (n = 59) . | Imaged (n = 250) . | No CV (n = 11) . | Total (n = 747) . | Active CV (n = 519) . | Spontaneous CV (n = 138) . | Imaged (n = 437) . | No CV (n = 90) . | |
| Strokes | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 3 | 1 | 3 | 2 | |
| Ischaemic | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 3 | 1 | 3 | 1 | |
| Haemorrhagic | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | |
| Systemic embolism | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Death | 2 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | |
| Safety outcomes (safety populationb; n = 1456) | ||||||||||||||||
Apixaban | Apixaban loading dose | Heparin/VKA | ||||||||||||||
| Total (n = 735) | Active (n = 520) | Spontaneous (n = 161) | Imaged (n = 417) | No CV (n = 54) | Total (n = 342) | Active (n = 272) | Spontaneous (n = 59) | Imaged (n = 250) | No CV (n = 11) | Total (n = 721) | Active (n = 517) | Spontaneous (n = 137) | Imaged (n = 436) | No CV (n = 67) | ||
| Major bleeds | 3 | 3 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 6 | 2 | 3 | 2 | 1 | |
| CRNM bleeds | 11 | 8 | 2 | 8 | 1 | 4 | 2 | 2 | 2 | 0 | 13 | 9 | 2 | 8 | 2 | |
| Efficacy outcomes (intention-to-treat populationa; N = 1500) . | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Apixaban (n = 753) . | Apixaban loading dose (n = 342) . | Heparin/VKA (n = 747) . | |||||||||||||
| . | Total (n = 753) . | Active CV (n = 519) . | Spontaneous CV (n = 162) . | Imaged (n = 418) . | No CV (n = 72) . | Total (n = 342) . | Active CV (n = 272) . | Spontaneous CV (n = 59) . | Imaged (n = 250) . | No CV (n = 11) . | Total (n = 747) . | Active CV (n = 519) . | Spontaneous CV (n = 138) . | Imaged (n = 437) . | No CV (n = 90) . | |
| Strokes | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 3 | 1 | 3 | 2 | |
| Ischaemic | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 3 | 1 | 3 | 1 | |
| Haemorrhagic | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | |
| Systemic embolism | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Death | 2 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | |
| Safety outcomes (safety populationb; n = 1456) | ||||||||||||||||
Apixaban | Apixaban loading dose | Heparin/VKA | ||||||||||||||
| Total (n = 735) | Active (n = 520) | Spontaneous (n = 161) | Imaged (n = 417) | No CV (n = 54) | Total (n = 342) | Active (n = 272) | Spontaneous (n = 59) | Imaged (n = 250) | No CV (n = 11) | Total (n = 721) | Active (n = 517) | Spontaneous (n = 137) | Imaged (n = 436) | No CV (n = 67) | ||
| Major bleeds | 3 | 3 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 6 | 2 | 3 | 2 | 1 | |
| CRNM bleeds | 11 | 8 | 2 | 8 | 1 | 4 | 2 | 2 | 2 | 0 | 13 | 9 | 2 | 8 | 2 | |
CRNM, clinically relevant non-major; CV, cardioversion; VKA, vitamin K antagonist.
All randomized.
Randomized and received at least one dose of study medication. Note: six patients received drug but not their assigned drug. The safety population is analysed according to the actual drug received.
Efficacy and safety outcomes
| Efficacy outcomes (intention-to-treat populationa; N = 1500) . | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Apixaban (n = 753) . | Apixaban loading dose (n = 342) . | Heparin/VKA (n = 747) . | |||||||||||||
| . | Total (n = 753) . | Active CV (n = 519) . | Spontaneous CV (n = 162) . | Imaged (n = 418) . | No CV (n = 72) . | Total (n = 342) . | Active CV (n = 272) . | Spontaneous CV (n = 59) . | Imaged (n = 250) . | No CV (n = 11) . | Total (n = 747) . | Active CV (n = 519) . | Spontaneous CV (n = 138) . | Imaged (n = 437) . | No CV (n = 90) . | |
| Strokes | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 3 | 1 | 3 | 2 | |
| Ischaemic | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 3 | 1 | 3 | 1 | |
| Haemorrhagic | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | |
| Systemic embolism | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Death | 2 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | |
| Safety outcomes (safety populationb; n = 1456) | ||||||||||||||||
Apixaban | Apixaban loading dose | Heparin/VKA | ||||||||||||||
| Total (n = 735) | Active (n = 520) | Spontaneous (n = 161) | Imaged (n = 417) | No CV (n = 54) | Total (n = 342) | Active (n = 272) | Spontaneous (n = 59) | Imaged (n = 250) | No CV (n = 11) | Total (n = 721) | Active (n = 517) | Spontaneous (n = 137) | Imaged (n = 436) | No CV (n = 67) | ||
| Major bleeds | 3 | 3 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 6 | 2 | 3 | 2 | 1 | |
| CRNM bleeds | 11 | 8 | 2 | 8 | 1 | 4 | 2 | 2 | 2 | 0 | 13 | 9 | 2 | 8 | 2 | |
| Efficacy outcomes (intention-to-treat populationa; N = 1500) . | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Apixaban (n = 753) . | Apixaban loading dose (n = 342) . | Heparin/VKA (n = 747) . | |||||||||||||
| . | Total (n = 753) . | Active CV (n = 519) . | Spontaneous CV (n = 162) . | Imaged (n = 418) . | No CV (n = 72) . | Total (n = 342) . | Active CV (n = 272) . | Spontaneous CV (n = 59) . | Imaged (n = 250) . | No CV (n = 11) . | Total (n = 747) . | Active CV (n = 519) . | Spontaneous CV (n = 138) . | Imaged (n = 437) . | No CV (n = 90) . | |
| Strokes | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 3 | 1 | 3 | 2 | |
| Ischaemic | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 3 | 1 | 3 | 1 | |
| Haemorrhagic | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | |
| Systemic embolism | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Death | 2 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | |
| Safety outcomes (safety populationb; n = 1456) | ||||||||||||||||
Apixaban | Apixaban loading dose | Heparin/VKA | ||||||||||||||
| Total (n = 735) | Active (n = 520) | Spontaneous (n = 161) | Imaged (n = 417) | No CV (n = 54) | Total (n = 342) | Active (n = 272) | Spontaneous (n = 59) | Imaged (n = 250) | No CV (n = 11) | Total (n = 721) | Active (n = 517) | Spontaneous (n = 137) | Imaged (n = 436) | No CV (n = 67) | ||
| Major bleeds | 3 | 3 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 6 | 2 | 3 | 2 | 1 | |
| CRNM bleeds | 11 | 8 | 2 | 8 | 1 | 4 | 2 | 2 | 2 | 0 | 13 | 9 | 2 | 8 | 2 | |
CRNM, clinically relevant non-major; CV, cardioversion; VKA, vitamin K antagonist.
All randomized.
Randomized and received at least one dose of study medication. Note: six patients received drug but not their assigned drug. The safety population is analysed according to the actual drug received.
Individual efficacy outcome events
| Events(s) . | Age/ gender . | Relevant medical history . | CHA2DS2- VASc Score . | Apixaban loading dose or INR/heparin . | Event day (days after randomization) . | Cardioversion (days after randomization) . | Cardioversion performed/ success . | Cardioversion type . | TOE performed . |
|---|---|---|---|---|---|---|---|---|---|
| Apixabana | |||||||||
| All-cause death | |||||||||
| Acute alcoholic hepatitis | 64/M | Alcoholic hepatitis | 0 | No | 0 | N/A | No | N/A | No |
| Perforated colitis | 81/M | CHF/CAD/Pacemaker/COPD/HTN/PE/PVD | 5 | Yes | 24 | 1 | Yes/Yes | E | Yes (no thrombus) |
| Heparin/VKA | |||||||||
| Strokes | |||||||||
| Ischaemic stroke | 69/F | DM/CHF/CVA/HTN/PCI | 7 | 20 | 0 | Yes/Yes | E | Yes (no thrombus) | |
| Ischaemic stroke with haemorrhagic conversion | 60/F | Bronchiectasis | 1 | 4 | N/A | No | N/A | No | |
| Haemorrhagic stroke | 77/M | HTN | 3 | 2.8 | 75 | N/A | No | N/A | No |
| Ischaemic stroke | 60/F | CHF (LVEF 15%) | 2 | 1.9 | 3 | 80 | Yes/Yes | E | Yes (no thrombus) |
| Ischaemic stroke | 75/M | Moderate aortic valve disease | 2 | Enoxaparin 150 mg QD | 8 | 1 | Yes/Yes | E | Yes (no thrombus) |
| Ischaemic stroke | 64/M | HTN | 1 | 2 | 1 | No/Yes | Spontaneous | No | |
| All-cause death | |||||||||
| Sudden death | 86/F | HTN/MI | 5 | 2 | 0 | Yes/Yes | E | No |
| Events(s) . | Age/ gender . | Relevant medical history . | CHA2DS2- VASc Score . | Apixaban loading dose or INR/heparin . | Event day (days after randomization) . | Cardioversion (days after randomization) . | Cardioversion performed/ success . | Cardioversion type . | TOE performed . |
|---|---|---|---|---|---|---|---|---|---|
| Apixabana | |||||||||
| All-cause death | |||||||||
| Acute alcoholic hepatitis | 64/M | Alcoholic hepatitis | 0 | No | 0 | N/A | No | N/A | No |
| Perforated colitis | 81/M | CHF/CAD/Pacemaker/COPD/HTN/PE/PVD | 5 | Yes | 24 | 1 | Yes/Yes | E | Yes (no thrombus) |
| Heparin/VKA | |||||||||
| Strokes | |||||||||
| Ischaemic stroke | 69/F | DM/CHF/CVA/HTN/PCI | 7 | 20 | 0 | Yes/Yes | E | Yes (no thrombus) | |
| Ischaemic stroke with haemorrhagic conversion | 60/F | Bronchiectasis | 1 | 4 | N/A | No | N/A | No | |
| Haemorrhagic stroke | 77/M | HTN | 3 | 2.8 | 75 | N/A | No | N/A | No |
| Ischaemic stroke | 60/F | CHF (LVEF 15%) | 2 | 1.9 | 3 | 80 | Yes/Yes | E | Yes (no thrombus) |
| Ischaemic stroke | 75/M | Moderate aortic valve disease | 2 | Enoxaparin 150 mg QD | 8 | 1 | Yes/Yes | E | Yes (no thrombus) |
| Ischaemic stroke | 64/M | HTN | 1 | 2 | 1 | No/Yes | Spontaneous | No | |
| All-cause death | |||||||||
| Sudden death | 86/F | HTN/MI | 5 | 2 | 0 | Yes/Yes | E | No |
No strokes.
CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CVA, cardiovascular disease; DM, diabetes mellitus; E, electrical; F, female; GI, gastro-intestinal; HTN, hypertension; PCI, percutaneous coronary intervention; PE, pulmonary embolism; PVD, peripheral vascular disease; LVEF, left ventricular ejection fraction; M, male; MI, myocardial infarction; N/A, not applicable.
Individual efficacy outcome events
| Events(s) . | Age/ gender . | Relevant medical history . | CHA2DS2- VASc Score . | Apixaban loading dose or INR/heparin . | Event day (days after randomization) . | Cardioversion (days after randomization) . | Cardioversion performed/ success . | Cardioversion type . | TOE performed . |
|---|---|---|---|---|---|---|---|---|---|
| Apixabana | |||||||||
| All-cause death | |||||||||
| Acute alcoholic hepatitis | 64/M | Alcoholic hepatitis | 0 | No | 0 | N/A | No | N/A | No |
| Perforated colitis | 81/M | CHF/CAD/Pacemaker/COPD/HTN/PE/PVD | 5 | Yes | 24 | 1 | Yes/Yes | E | Yes (no thrombus) |
| Heparin/VKA | |||||||||
| Strokes | |||||||||
| Ischaemic stroke | 69/F | DM/CHF/CVA/HTN/PCI | 7 | 20 | 0 | Yes/Yes | E | Yes (no thrombus) | |
| Ischaemic stroke with haemorrhagic conversion | 60/F | Bronchiectasis | 1 | 4 | N/A | No | N/A | No | |
| Haemorrhagic stroke | 77/M | HTN | 3 | 2.8 | 75 | N/A | No | N/A | No |
| Ischaemic stroke | 60/F | CHF (LVEF 15%) | 2 | 1.9 | 3 | 80 | Yes/Yes | E | Yes (no thrombus) |
| Ischaemic stroke | 75/M | Moderate aortic valve disease | 2 | Enoxaparin 150 mg QD | 8 | 1 | Yes/Yes | E | Yes (no thrombus) |
| Ischaemic stroke | 64/M | HTN | 1 | 2 | 1 | No/Yes | Spontaneous | No | |
| All-cause death | |||||||||
| Sudden death | 86/F | HTN/MI | 5 | 2 | 0 | Yes/Yes | E | No |
| Events(s) . | Age/ gender . | Relevant medical history . | CHA2DS2- VASc Score . | Apixaban loading dose or INR/heparin . | Event day (days after randomization) . | Cardioversion (days after randomization) . | Cardioversion performed/ success . | Cardioversion type . | TOE performed . |
|---|---|---|---|---|---|---|---|---|---|
| Apixabana | |||||||||
| All-cause death | |||||||||
| Acute alcoholic hepatitis | 64/M | Alcoholic hepatitis | 0 | No | 0 | N/A | No | N/A | No |
| Perforated colitis | 81/M | CHF/CAD/Pacemaker/COPD/HTN/PE/PVD | 5 | Yes | 24 | 1 | Yes/Yes | E | Yes (no thrombus) |
| Heparin/VKA | |||||||||
| Strokes | |||||||||
| Ischaemic stroke | 69/F | DM/CHF/CVA/HTN/PCI | 7 | 20 | 0 | Yes/Yes | E | Yes (no thrombus) | |
| Ischaemic stroke with haemorrhagic conversion | 60/F | Bronchiectasis | 1 | 4 | N/A | No | N/A | No | |
| Haemorrhagic stroke | 77/M | HTN | 3 | 2.8 | 75 | N/A | No | N/A | No |
| Ischaemic stroke | 60/F | CHF (LVEF 15%) | 2 | 1.9 | 3 | 80 | Yes/Yes | E | Yes (no thrombus) |
| Ischaemic stroke | 75/M | Moderate aortic valve disease | 2 | Enoxaparin 150 mg QD | 8 | 1 | Yes/Yes | E | Yes (no thrombus) |
| Ischaemic stroke | 64/M | HTN | 1 | 2 | 1 | No/Yes | Spontaneous | No | |
| All-cause death | |||||||||
| Sudden death | 86/F | HTN/MI | 5 | 2 | 0 | Yes/Yes | E | No |
No strokes.
CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CVA, cardiovascular disease; DM, diabetes mellitus; E, electrical; F, female; GI, gastro-intestinal; HTN, hypertension; PCI, percutaneous coronary intervention; PE, pulmonary embolism; PVD, peripheral vascular disease; LVEF, left ventricular ejection fraction; M, male; MI, myocardial infarction; N/A, not applicable.
Individual safety outcome events (Apixaban)
| Events . | Age/ gender . | Relevant medical history . | CHA2DS2- VASc score . | Apixaban loading dose . | Event day (days after randomization) . | Cardioversion day (days after randomization) . | Cardioversion performed/ success . | Cardioversion type . | Image guidance performed . |
|---|---|---|---|---|---|---|---|---|---|
| Major bleeds | |||||||||
| GI bleed, non-fatal | 68/M | HTN, ischaemic heart disease | 2 | No | 33 | 1 | Yes/Yes | Pharm | Yes (no thrombus) |
| GI bleed, non-fatal | 74/F | COPD | 2 | No | 20 | 63 | Yes/Yes | E | No |
| GI bleed, non-fatal | 74/F | DM/HTN | 4 | Yes | 17 | 0 | Yes/Yes | E | No |
| CRNM bleeds | |||||||||
| Epistaxis | 64/F | HTN | 2 | Yes | 14 | 1 | No/Yes | Spontaneous | No |
| GI bleed | 76/M | HTN/COPD | 3 | Yes | 11 | 1 | Yes/Yes | E | Yes (no thrombus) |
| Haematuria | 73/M | DM/HTN/CHF/PAH | 4 | No | 1 | N/A | No | N/A | Yes (no thrombus) |
| Haematuria | 88/F | HTN/MI/COPD/CAD | 5 | No | 26 | 13 | Yes/Yes | E | Yes (no thrombus) |
| Post-operative arterial haemorrhage | 52/M | HTN/COPD | 1 | Yes | 16 | 0 | No/Yes | Spontaneous | No |
| Haematoma | 69/M | Asthma | 1 | No | 38 | 30 | Yes/Yes | E | Yes (no thrombus) |
| Haematuria | 79/M | HTN/CAD/renal insufficiency | 4 | No | 21 | 4 | Yes/Yes | E | Yes (no thrombus) |
| Adrenal haemorrhage | 58/M | HTN | 1 | Yes | 15 | 1 | Yes/Yes | E | Yes (no thrombus) |
| Haematuria | 69/M | DM/HTN | 3 | No | 5 | 52 | Yes/Yes | E | No |
| Haematuria | 68/F | HTN/prior DVT | 3 | No | 0 | 48 | Yes/Yes | E | Yes (no thrombus) |
| Traumatic haemorrhage | 74/M | HTN/aortic dissection | 2 | No | 22 | 29 | Yes/Yes | E | Yes (no thrombus) |
| Events . | Age/ gender . | Relevant medical history . | CHA2DS2- VASc score . | Apixaban loading dose . | Event day (days after randomization) . | Cardioversion day (days after randomization) . | Cardioversion performed/ success . | Cardioversion type . | Image guidance performed . |
|---|---|---|---|---|---|---|---|---|---|
| Major bleeds | |||||||||
| GI bleed, non-fatal | 68/M | HTN, ischaemic heart disease | 2 | No | 33 | 1 | Yes/Yes | Pharm | Yes (no thrombus) |
| GI bleed, non-fatal | 74/F | COPD | 2 | No | 20 | 63 | Yes/Yes | E | No |
| GI bleed, non-fatal | 74/F | DM/HTN | 4 | Yes | 17 | 0 | Yes/Yes | E | No |
| CRNM bleeds | |||||||||
| Epistaxis | 64/F | HTN | 2 | Yes | 14 | 1 | No/Yes | Spontaneous | No |
| GI bleed | 76/M | HTN/COPD | 3 | Yes | 11 | 1 | Yes/Yes | E | Yes (no thrombus) |
| Haematuria | 73/M | DM/HTN/CHF/PAH | 4 | No | 1 | N/A | No | N/A | Yes (no thrombus) |
| Haematuria | 88/F | HTN/MI/COPD/CAD | 5 | No | 26 | 13 | Yes/Yes | E | Yes (no thrombus) |
| Post-operative arterial haemorrhage | 52/M | HTN/COPD | 1 | Yes | 16 | 0 | No/Yes | Spontaneous | No |
| Haematoma | 69/M | Asthma | 1 | No | 38 | 30 | Yes/Yes | E | Yes (no thrombus) |
| Haematuria | 79/M | HTN/CAD/renal insufficiency | 4 | No | 21 | 4 | Yes/Yes | E | Yes (no thrombus) |
| Adrenal haemorrhage | 58/M | HTN | 1 | Yes | 15 | 1 | Yes/Yes | E | Yes (no thrombus) |
| Haematuria | 69/M | DM/HTN | 3 | No | 5 | 52 | Yes/Yes | E | No |
| Haematuria | 68/F | HTN/prior DVT | 3 | No | 0 | 48 | Yes/Yes | E | Yes (no thrombus) |
| Traumatic haemorrhage | 74/M | HTN/aortic dissection | 2 | No | 22 | 29 | Yes/Yes | E | Yes (no thrombus) |
CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CV, cardioversion; DM, diabetes mellitus; DVT, deep vein thrombosis; E, electrical; F, female; GI, gastro-intestinal; HTN, hypertension; M, male; MI, myocardial infarction; N/A, not applicable; PAH, pulmonary hypertension.
Individual safety outcome events (Apixaban)
| Events . | Age/ gender . | Relevant medical history . | CHA2DS2- VASc score . | Apixaban loading dose . | Event day (days after randomization) . | Cardioversion day (days after randomization) . | Cardioversion performed/ success . | Cardioversion type . | Image guidance performed . |
|---|---|---|---|---|---|---|---|---|---|
| Major bleeds | |||||||||
| GI bleed, non-fatal | 68/M | HTN, ischaemic heart disease | 2 | No | 33 | 1 | Yes/Yes | Pharm | Yes (no thrombus) |
| GI bleed, non-fatal | 74/F | COPD | 2 | No | 20 | 63 | Yes/Yes | E | No |
| GI bleed, non-fatal | 74/F | DM/HTN | 4 | Yes | 17 | 0 | Yes/Yes | E | No |
| CRNM bleeds | |||||||||
| Epistaxis | 64/F | HTN | 2 | Yes | 14 | 1 | No/Yes | Spontaneous | No |
| GI bleed | 76/M | HTN/COPD | 3 | Yes | 11 | 1 | Yes/Yes | E | Yes (no thrombus) |
| Haematuria | 73/M | DM/HTN/CHF/PAH | 4 | No | 1 | N/A | No | N/A | Yes (no thrombus) |
| Haematuria | 88/F | HTN/MI/COPD/CAD | 5 | No | 26 | 13 | Yes/Yes | E | Yes (no thrombus) |
| Post-operative arterial haemorrhage | 52/M | HTN/COPD | 1 | Yes | 16 | 0 | No/Yes | Spontaneous | No |
| Haematoma | 69/M | Asthma | 1 | No | 38 | 30 | Yes/Yes | E | Yes (no thrombus) |
| Haematuria | 79/M | HTN/CAD/renal insufficiency | 4 | No | 21 | 4 | Yes/Yes | E | Yes (no thrombus) |
| Adrenal haemorrhage | 58/M | HTN | 1 | Yes | 15 | 1 | Yes/Yes | E | Yes (no thrombus) |
| Haematuria | 69/M | DM/HTN | 3 | No | 5 | 52 | Yes/Yes | E | No |
| Haematuria | 68/F | HTN/prior DVT | 3 | No | 0 | 48 | Yes/Yes | E | Yes (no thrombus) |
| Traumatic haemorrhage | 74/M | HTN/aortic dissection | 2 | No | 22 | 29 | Yes/Yes | E | Yes (no thrombus) |
| Events . | Age/ gender . | Relevant medical history . | CHA2DS2- VASc score . | Apixaban loading dose . | Event day (days after randomization) . | Cardioversion day (days after randomization) . | Cardioversion performed/ success . | Cardioversion type . | Image guidance performed . |
|---|---|---|---|---|---|---|---|---|---|
| Major bleeds | |||||||||
| GI bleed, non-fatal | 68/M | HTN, ischaemic heart disease | 2 | No | 33 | 1 | Yes/Yes | Pharm | Yes (no thrombus) |
| GI bleed, non-fatal | 74/F | COPD | 2 | No | 20 | 63 | Yes/Yes | E | No |
| GI bleed, non-fatal | 74/F | DM/HTN | 4 | Yes | 17 | 0 | Yes/Yes | E | No |
| CRNM bleeds | |||||||||
| Epistaxis | 64/F | HTN | 2 | Yes | 14 | 1 | No/Yes | Spontaneous | No |
| GI bleed | 76/M | HTN/COPD | 3 | Yes | 11 | 1 | Yes/Yes | E | Yes (no thrombus) |
| Haematuria | 73/M | DM/HTN/CHF/PAH | 4 | No | 1 | N/A | No | N/A | Yes (no thrombus) |
| Haematuria | 88/F | HTN/MI/COPD/CAD | 5 | No | 26 | 13 | Yes/Yes | E | Yes (no thrombus) |
| Post-operative arterial haemorrhage | 52/M | HTN/COPD | 1 | Yes | 16 | 0 | No/Yes | Spontaneous | No |
| Haematoma | 69/M | Asthma | 1 | No | 38 | 30 | Yes/Yes | E | Yes (no thrombus) |
| Haematuria | 79/M | HTN/CAD/renal insufficiency | 4 | No | 21 | 4 | Yes/Yes | E | Yes (no thrombus) |
| Adrenal haemorrhage | 58/M | HTN | 1 | Yes | 15 | 1 | Yes/Yes | E | Yes (no thrombus) |
| Haematuria | 69/M | DM/HTN | 3 | No | 5 | 52 | Yes/Yes | E | No |
| Haematuria | 68/F | HTN/prior DVT | 3 | No | 0 | 48 | Yes/Yes | E | Yes (no thrombus) |
| Traumatic haemorrhage | 74/M | HTN/aortic dissection | 2 | No | 22 | 29 | Yes/Yes | E | Yes (no thrombus) |
CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CV, cardioversion; DM, diabetes mellitus; DVT, deep vein thrombosis; E, electrical; F, female; GI, gastro-intestinal; HTN, hypertension; M, male; MI, myocardial infarction; N/A, not applicable; PAH, pulmonary hypertension.
Individual safety outcome events (heparin/VKA)
| Events . | Age/ gender . | Relevant medical history . | CHA2DS2- VASc Score . | INR . | Event day (days after randomization) . | Cardioversion day (days after randomization) . | Cardioversion performed/ success . | Cardioversion type . | Image guidance performed . |
|---|---|---|---|---|---|---|---|---|---|
| Major bleeds | |||||||||
| GI bleed, non-fatal | 83/M | DM/HTN/CAD/PAH | 5 | 21 | 1 | Yes/Yes | E | Yes (no thrombus) | |
| Retroperitoneal, non-fatal | 57/M | None | 0 | 4.3 | 27 | 0 | No/Yes | Spontaneous | No |
| Haemorrhagic stroke, non-fatal | 77/M | HTN | 3 | 2.8, 1.4, 1.2 | 75 | N/A | No | N/A | No |
| GI bleed, non-fatal | 69/M | None | 1 | 1.7 | 8 | 0 | Yes/Yes | E | Yes (no thrombus) |
| GI bleed, non-fatal | 69/F | HTN/CHF/CAD | 5 | 1.4 | 18 | 43 | No/Yes | Spontaneous | No |
| GI bleed, non-fatal | 67M | HTN/CHF/MI/CAD | 4 | 4.8 | 11 | 0 | No/Yes | Spontaneous | No |
| CRNM bleeds | |||||||||
| GI bleed | 79/M | HTN/CAD/PAD | 4 | 1 | 0 | Yes/Yes | E | Yes (no thrombus) | |
| Vaginal bleed | 70/F | HTN/CAD/stroke | 6 | 3.7 | 4 | 0 | Yes/Yes | E | Yes (no thrombus) |
| Haematoma | 69/F | DM/HTN/CHF/stroke | 7 | 4 | 0 | Yes/Yes | E | Yes (no thrombus) | |
| Vaginal bleed | 54/F | None | 1 | 15 | 1 | Yes/Yes | E | Yes (no thrombus) | |
| Epistaxis | 77/M | HTN | 3 | 3.6 | 32 | N/A | No | N/A | No |
| GI bleed | 72/M | HTN | 2 | 88 | 5 | No/Yes | Spontaneous | Yes (no thrombus) | |
| GI bleed | 36/M | None | 0 | 1.5 | 5 | 0 | Yes/Yes | E | Yes (no thrombus) |
| Haematuria | 63/M | HTN | 1 | 2.0 | 30 | 65 | Yes/Yes | E | Yes (no thrombus) |
| GI bleed | 84/F | None | 3 | 57 | 57 | Yes/Yes | E | No | |
| Haematoma | 66/F | None | 2 | 1.4 | 14 | N/A | No | N/A | No |
| Hyposphagma | 88/M | HTN/CHF | 4 | 31 | 3 | No/Yes | Spontaneous | No | |
| Haematuria | 73/M | HTN/CAD | 3 | 3.8 | 21 | 15 | Yes/Yes | E | Yes (no thrombus) |
| Epistaxis | 58/M | HTN/CHF/CAD/renal insuff | 3 | 2.6 | 1 | 0 | Yes/No | E | Yes (no thrombus) |
| Events . | Age/ gender . | Relevant medical history . | CHA2DS2- VASc Score . | INR . | Event day (days after randomization) . | Cardioversion day (days after randomization) . | Cardioversion performed/ success . | Cardioversion type . | Image guidance performed . |
|---|---|---|---|---|---|---|---|---|---|
| Major bleeds | |||||||||
| GI bleed, non-fatal | 83/M | DM/HTN/CAD/PAH | 5 | 21 | 1 | Yes/Yes | E | Yes (no thrombus) | |
| Retroperitoneal, non-fatal | 57/M | None | 0 | 4.3 | 27 | 0 | No/Yes | Spontaneous | No |
| Haemorrhagic stroke, non-fatal | 77/M | HTN | 3 | 2.8, 1.4, 1.2 | 75 | N/A | No | N/A | No |
| GI bleed, non-fatal | 69/M | None | 1 | 1.7 | 8 | 0 | Yes/Yes | E | Yes (no thrombus) |
| GI bleed, non-fatal | 69/F | HTN/CHF/CAD | 5 | 1.4 | 18 | 43 | No/Yes | Spontaneous | No |
| GI bleed, non-fatal | 67M | HTN/CHF/MI/CAD | 4 | 4.8 | 11 | 0 | No/Yes | Spontaneous | No |
| CRNM bleeds | |||||||||
| GI bleed | 79/M | HTN/CAD/PAD | 4 | 1 | 0 | Yes/Yes | E | Yes (no thrombus) | |
| Vaginal bleed | 70/F | HTN/CAD/stroke | 6 | 3.7 | 4 | 0 | Yes/Yes | E | Yes (no thrombus) |
| Haematoma | 69/F | DM/HTN/CHF/stroke | 7 | 4 | 0 | Yes/Yes | E | Yes (no thrombus) | |
| Vaginal bleed | 54/F | None | 1 | 15 | 1 | Yes/Yes | E | Yes (no thrombus) | |
| Epistaxis | 77/M | HTN | 3 | 3.6 | 32 | N/A | No | N/A | No |
| GI bleed | 72/M | HTN | 2 | 88 | 5 | No/Yes | Spontaneous | Yes (no thrombus) | |
| GI bleed | 36/M | None | 0 | 1.5 | 5 | 0 | Yes/Yes | E | Yes (no thrombus) |
| Haematuria | 63/M | HTN | 1 | 2.0 | 30 | 65 | Yes/Yes | E | Yes (no thrombus) |
| GI bleed | 84/F | None | 3 | 57 | 57 | Yes/Yes | E | No | |
| Haematoma | 66/F | None | 2 | 1.4 | 14 | N/A | No | N/A | No |
| Hyposphagma | 88/M | HTN/CHF | 4 | 31 | 3 | No/Yes | Spontaneous | No | |
| Haematuria | 73/M | HTN/CAD | 3 | 3.8 | 21 | 15 | Yes/Yes | E | Yes (no thrombus) |
| Epistaxis | 58/M | HTN/CHF/CAD/renal insuff | 3 | 2.6 | 1 | 0 | Yes/No | E | Yes (no thrombus) |
CAD, coronary artery disease; CHF, congestive heart failure; DM, diabetes mellitus; E, electrical; F, female; GI, gastro-intestinal; HTN, hypertension; PVD, peripheral vascular disease; M, male; N/A, not applicable.
Individual safety outcome events (heparin/VKA)
| Events . | Age/ gender . | Relevant medical history . | CHA2DS2- VASc Score . | INR . | Event day (days after randomization) . | Cardioversion day (days after randomization) . | Cardioversion performed/ success . | Cardioversion type . | Image guidance performed . |
|---|---|---|---|---|---|---|---|---|---|
| Major bleeds | |||||||||
| GI bleed, non-fatal | 83/M | DM/HTN/CAD/PAH | 5 | 21 | 1 | Yes/Yes | E | Yes (no thrombus) | |
| Retroperitoneal, non-fatal | 57/M | None | 0 | 4.3 | 27 | 0 | No/Yes | Spontaneous | No |
| Haemorrhagic stroke, non-fatal | 77/M | HTN | 3 | 2.8, 1.4, 1.2 | 75 | N/A | No | N/A | No |
| GI bleed, non-fatal | 69/M | None | 1 | 1.7 | 8 | 0 | Yes/Yes | E | Yes (no thrombus) |
| GI bleed, non-fatal | 69/F | HTN/CHF/CAD | 5 | 1.4 | 18 | 43 | No/Yes | Spontaneous | No |
| GI bleed, non-fatal | 67M | HTN/CHF/MI/CAD | 4 | 4.8 | 11 | 0 | No/Yes | Spontaneous | No |
| CRNM bleeds | |||||||||
| GI bleed | 79/M | HTN/CAD/PAD | 4 | 1 | 0 | Yes/Yes | E | Yes (no thrombus) | |
| Vaginal bleed | 70/F | HTN/CAD/stroke | 6 | 3.7 | 4 | 0 | Yes/Yes | E | Yes (no thrombus) |
| Haematoma | 69/F | DM/HTN/CHF/stroke | 7 | 4 | 0 | Yes/Yes | E | Yes (no thrombus) | |
| Vaginal bleed | 54/F | None | 1 | 15 | 1 | Yes/Yes | E | Yes (no thrombus) | |
| Epistaxis | 77/M | HTN | 3 | 3.6 | 32 | N/A | No | N/A | No |
| GI bleed | 72/M | HTN | 2 | 88 | 5 | No/Yes | Spontaneous | Yes (no thrombus) | |
| GI bleed | 36/M | None | 0 | 1.5 | 5 | 0 | Yes/Yes | E | Yes (no thrombus) |
| Haematuria | 63/M | HTN | 1 | 2.0 | 30 | 65 | Yes/Yes | E | Yes (no thrombus) |
| GI bleed | 84/F | None | 3 | 57 | 57 | Yes/Yes | E | No | |
| Haematoma | 66/F | None | 2 | 1.4 | 14 | N/A | No | N/A | No |
| Hyposphagma | 88/M | HTN/CHF | 4 | 31 | 3 | No/Yes | Spontaneous | No | |
| Haematuria | 73/M | HTN/CAD | 3 | 3.8 | 21 | 15 | Yes/Yes | E | Yes (no thrombus) |
| Epistaxis | 58/M | HTN/CHF/CAD/renal insuff | 3 | 2.6 | 1 | 0 | Yes/No | E | Yes (no thrombus) |
| Events . | Age/ gender . | Relevant medical history . | CHA2DS2- VASc Score . | INR . | Event day (days after randomization) . | Cardioversion day (days after randomization) . | Cardioversion performed/ success . | Cardioversion type . | Image guidance performed . |
|---|---|---|---|---|---|---|---|---|---|
| Major bleeds | |||||||||
| GI bleed, non-fatal | 83/M | DM/HTN/CAD/PAH | 5 | 21 | 1 | Yes/Yes | E | Yes (no thrombus) | |
| Retroperitoneal, non-fatal | 57/M | None | 0 | 4.3 | 27 | 0 | No/Yes | Spontaneous | No |
| Haemorrhagic stroke, non-fatal | 77/M | HTN | 3 | 2.8, 1.4, 1.2 | 75 | N/A | No | N/A | No |
| GI bleed, non-fatal | 69/M | None | 1 | 1.7 | 8 | 0 | Yes/Yes | E | Yes (no thrombus) |
| GI bleed, non-fatal | 69/F | HTN/CHF/CAD | 5 | 1.4 | 18 | 43 | No/Yes | Spontaneous | No |
| GI bleed, non-fatal | 67M | HTN/CHF/MI/CAD | 4 | 4.8 | 11 | 0 | No/Yes | Spontaneous | No |
| CRNM bleeds | |||||||||
| GI bleed | 79/M | HTN/CAD/PAD | 4 | 1 | 0 | Yes/Yes | E | Yes (no thrombus) | |
| Vaginal bleed | 70/F | HTN/CAD/stroke | 6 | 3.7 | 4 | 0 | Yes/Yes | E | Yes (no thrombus) |
| Haematoma | 69/F | DM/HTN/CHF/stroke | 7 | 4 | 0 | Yes/Yes | E | Yes (no thrombus) | |
| Vaginal bleed | 54/F | None | 1 | 15 | 1 | Yes/Yes | E | Yes (no thrombus) | |
| Epistaxis | 77/M | HTN | 3 | 3.6 | 32 | N/A | No | N/A | No |
| GI bleed | 72/M | HTN | 2 | 88 | 5 | No/Yes | Spontaneous | Yes (no thrombus) | |
| GI bleed | 36/M | None | 0 | 1.5 | 5 | 0 | Yes/Yes | E | Yes (no thrombus) |
| Haematuria | 63/M | HTN | 1 | 2.0 | 30 | 65 | Yes/Yes | E | Yes (no thrombus) |
| GI bleed | 84/F | None | 3 | 57 | 57 | Yes/Yes | E | No | |
| Haematoma | 66/F | None | 2 | 1.4 | 14 | N/A | No | N/A | No |
| Hyposphagma | 88/M | HTN/CHF | 4 | 31 | 3 | No/Yes | Spontaneous | No | |
| Haematuria | 73/M | HTN/CAD | 3 | 3.8 | 21 | 15 | Yes/Yes | E | Yes (no thrombus) |
| Epistaxis | 58/M | HTN/CHF/CAD/renal insuff | 3 | 2.6 | 1 | 0 | Yes/No | E | Yes (no thrombus) |
CAD, coronary artery disease; CHF, congestive heart failure; DM, diabetes mellitus; E, electrical; F, female; GI, gastro-intestinal; HTN, hypertension; PVD, peripheral vascular disease; M, male; N/A, not applicable.
Imaging
Pre-procedural imaging was obtained in 855 patients. Of these, 829 underwent TOE, 14 CT imaging and 1 patient had both. The type of the imaging was not specified for the remaining 11 patients. Sixty-one patients with imaging had left atrial thrombus, 30 in the apixaban group and 31 in the heparin/VKA group (Figure 4). Sixty continued assigned medication while 1 patient in the apixaban group switched to heparin/VKA. In the apixaban group, 23/30 (77%) underwent repeat imaging with resolution of thrombus in 12 (52%). In the heparin/VKA group, 18/31 (58%) patients were re-imaged and thrombus resolved in 10 (56%) (Figure 4, Tables 3, 4, 5, and 6).
Seven patients with left atrial thrombi withdrew from the study; four assigned to apixaban and three to heparin/vitamin K antagonist. Follow-up from randomization to patient withdrawal was 8, 36, 1, and 31 days in the apixaban arm and 73, 67, 27 in the heparin/vitamin K antagonist arm, respectively. One patient was randomized to apixaban and transferred to usual care and withdrawn on Day 1. There were no cardioversions performed. For the whole group of 61 patients with left atrial thrombi, there were no outcome events. SD, standard deviation; TOE, transoesophageal echocardiogram; VKA, vitamin K antagonist.
Seven patients with left atrial thrombi withdrew from the study; 4 assigned to apixaban and 3 to heparin/VKA. Follow-up from randomization to patient withdrawal was 1, 8, 31, and 36 days in the apixaban arm and 27, 67, and 73 days in the heparin/VKA arm, respectively. One patient was randomized to apixaban and transferred to usual care and withdrawn on Day 1. There were no cardioversions performed in these 7 patients who withdrew. In patients with left atrial or left atrial appendage thrombus, no strokes, SE, major bleeding, or deaths occurred in either treatment group.
Loading dose and imaging
The mean time from the loading dose of apixaban to first active cardioversion was 3.3 days (SD = 8.8) in those undergoing imaging and 4.1 days (SD = 9.5) without imaging. In patients who did not receive a loading dose, the mean time to first cardioversion was 21.7 days (SD = 21.2) with imaging and 32.5 days (SD = 14.6) without imaging (Table 2). In the heparin/VKA group, the mean time from first dose to cardioversion was 17.8 days (SD = 24.7) (Table 2).
Cardioverted groups
Of the 925 patients undergoing a first electrical cardioversion, there were no strokes in the apixaban group vs. 3 in the heparin/VKA group. There were 2 major bleeding events in each group, and 8 vs. 9 CRNM haemorrhages in the apixaban and heparin/VKA groups, respectively. There was 1 death in each arm and no SE events in either group (Table 3). Among 65 patients undergoing pharmacological cardioversion, there were no stroke, SE, or fatal events but 1 major bleed in the apixaban group. Fifty-two patients were converted both pharmacologically and electrically without outcome events. For patients undergoing pharmacologic cardioversion alone or together with electrical cardioversion, the most commonly used antiarrhythmic agents were amiodarone (57%), propafenone (18%), and flecainide (13%) (Table 1).
Of the 300 patients converting spontaneously, there were no strokes or major bleeds in the apixaban group vs. 1 stroke and 3 major bleeds in the heparin/VKA group and 2 CRNM bleeds in each group. Among 162 patients not cardioverted, there were 2 strokes (1 ischaemic and 1 haemorrhagic) in patients randomized to heparin/VKA (Table 3).
Serious adverse events
Serious adverse events occurred in 100 (14%) of apixaban-treated patients and 112 (16%) of patients treated with heparin/VKA; the most common (≥1% of patients) events were AF (27 and 40 patients, respectively) and heart failure (9 and 7 patients, respectively). There were 3 myocardial infarctions, 2 on apixaban occurring on Days 2 and 26 after dosing, and 1 on heparin/VKA on the first day prior to dosing. None were fatal.
Discussion
EMANATE trial differed from the two other completed randomized trials testing a factor Xa inhibitor by including only patients with minimal exposure to anticoagulation prior to randomization with 62% receiving no anticoagulation and 38% ≤ 48 h of anticoagulation. In addition, 78% had new onset or AF defined as AF first diagnosed within 3 months prior to randomization. In other respects, the three trials were similar with low event rates and with treatment assignment randomized by protocol. In EMANATE the protocol encouraged, but did not mandate, imaging or the type of imaging to use, leaving the decision to the discretion of the investigator. Likewise, the use of the loading dose option in those randomized to apixaban was at the investigator’s discretion. We believe the results of EMANATE are clinically important and justify the use of apixaban in the setting of early and elective cardioversion in patients with AF of predominantly recent onset and can simplify the management of these patients compared to heparin/VKA.
Like two recent trials evaluating the other factor Xa inhibitors, EMANATE was an exploratory but clinically meaningful study. A sample size of approximately 48 000 patients would be needed to power for non-inferiority. In all three prospective trials of cardioversion, the heparin/VKA arms had similar and low event rates of outcome measures demonstrating consistency across the trials.12 , 13 The EMANATE trial unlike the other trials collected data on CRNM bleeds. There was no difference in the two randomized groups. Statistical methodologies used in the trials were similar. Low event rates reflect remarkable advances in cardiovascular medicine over the past 30 years. While low event rates are highly desirable for patients, they create difficulty creating large enough trials to have enough endpoint events to conduct meaningful statistical analysis. Together, these studies provide important data suggesting that all factor Xa inhibitors are viable alternatives to heparin/VKA in AF patients undergoing cardioversion.
The EMANATE study had features designed to reflect routine clinical practice. Although treatment was randomized, anticoagulation treatment was open-label. Whereas apixaban was provided by the sponsor, usual care was sourced locally with commercial drug supply provided using the investigator site with no packaging or labelling performed unless required by local country regulation. The protocol allowed cardioversion 2 h after dosing with use of imaging and a loading dose of apixaban. Among the 342 patients who received a loading dose of apixaban there were very few outcome events: no strokes, one major bleeding event, 4 CRNM bleeding events, and one death.
EMANATE trial adds to the insights from the X-TRA study and the CLOT- AF registry by systematically evaluating the use of a non-VKA in the setting of image-identified left atrial thrombus.21 Resolution of thrombi in patients receiving either apixaban or heparin/VKA was 52% and 58% respectively as determined by the local investigator based on repeated imaging done at 37 ± 11 days (mean ± SD) after the first images were obtained. In the group of 61 patients with a thrombus, 60 continued their allocated anticoagulant and there was complete follow-up and no outcome events (Figure 4).
The major limitation of EMANATE was that the study was descriptive. There was no hypothesis testing and no power calculations. The sample size of 1500 patients was considered clinically meaningful and achievable, similar to the X-VeRT trial.12 Additionally, while we cannot exclude that a few of the 28 patients who withdrew consent or that the single patient lost to follow-up could have experienced an outcome event, we believe it is unlikely that the patients that withdrew consent could have materially changed the result of the study. Nine patients withdrew without receiving medication while 16 others (apixaban:heparin/VKA 8:8) were not actively cardioverted and were followed for a mean of 31.7 days, all without an outcome event. Three patients were actively cardioverted (two randomized to apixaban, one to heparin/VKA) and were followed for a mean of 9.7 days post-cardioversion without an outcome event.
In summary, the data collected from the EMANATE trial of 1500 patients, with predominantly new onset AF undergoing elective cardioversion, found low stroke, systemic embolic events, death and bleeding events in both the apixaban and heparin/VKA-treated patients.
Footnotes
See page 2972 for the editorial comment on this article (doi: 10.1093/eurheartj/ehy303)
Acknowledgements
The content of this manuscript was developed solely by the authors. Editorial support, in the form of formatting the manuscript according to journal guidelines, was provided by Christopher Trent-Davis; Caudex Medical, funded by Pfizer and Bristol-Myers Squibb, provided additional editorial support and confirmed the accuracy of the data.
Funding
EMANATE was supported by Pfizer and Bristol-Myers Squibb.
Conflict of interest: M.D.E. reports receiving consulting fees from Boehringer Ingelheim, Armetheon, Pfizer, Sanofi, Bristol-Myers Squibb, Portola, Daiichi-Sankyo, Medtronics, Johnson & Johnson, and Janssen Scientific Affairs and grant support from Boehringer Ingelheim and Pfizer; he chaired the EMANATE Executive Committee, served as an Executive Committee member of Ensure-AF and was Co-Principal Investigator of the X-VeRT trial. C.V.P. reports receiving research support from Boehringer Ingelheim, Janssen, Daiichi-Sankyo, Portola, CSL Behring, and AstraZeneca and consulting fees from Boehringer Ingelheim, Janssen, Bristol-Myers Squibb/Pfizer, and Portola and served on the Executive Committee of EMANATE. J.L.H. reports receiving consulting fees from Bayer AG HealthCare, Boehringer Ingelheim, Johnson & Johnson, Ortho-McNeil-Janssen Pharmaceuticals and Pfizer. He was a member of the Executive Committee of EMANATE. R.D.E., S.V.N., M.S., N.B.C., and A.B. report being employees of Pfizer. R.D.E., N.B.C., and A.B. were members of the executive committee of EMANATE. J.S. reports receiving consulting fees from Pfizer. She was a member of the Executive Committee of EMANATE. J.O. reports that his institution received consulting and lecture fees from Bayer, Boehringer‐Ingelheim, Bristol‐Myers Squibb, Pfizer and Sanofi and was a member of the Executive Committee of EMANATE. P.K. reports research support from European Union, British Heart Foundation, Leducq Foundation, Medical Research Council (UK), and German Centre for Heart Research, from several drug and device companies active in atrial fibrillation, and has received honoraria from several such companies including Pfizer. He served on the Executive Committee of EMANATE. In addition, P.K. is listed as inventor on two patents held by University of Birmingham (Atrial Fibrillation Therapy WO 2015140571, Markers for Atrial Fibrillation WO 2016012783). No other potential conflict of interest relevant to this article was reported.
References



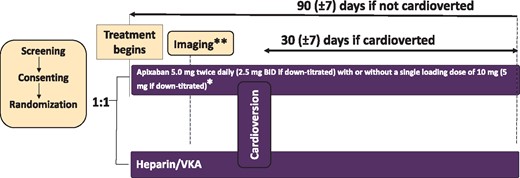
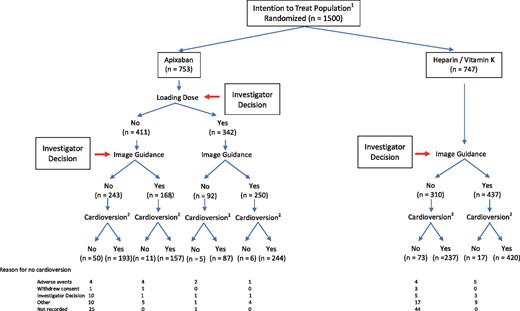
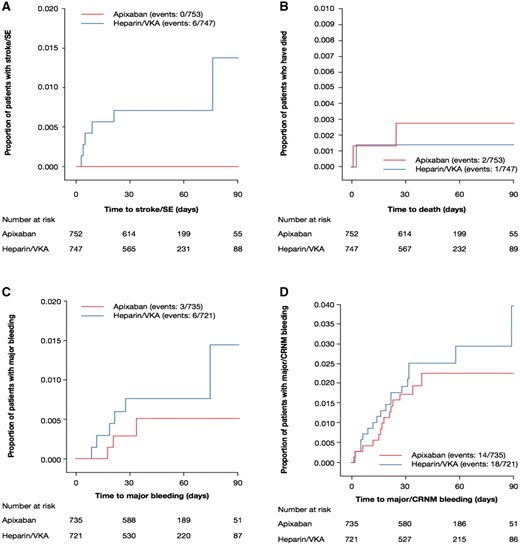
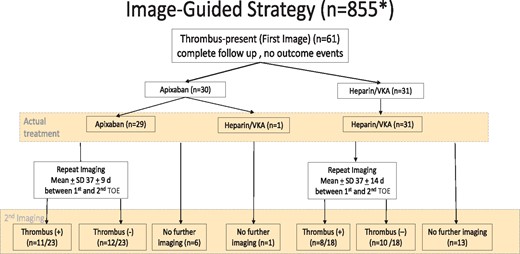

Comments
I read with interest the study by Ezekowitz et al. The issue of optimal timing of cardioversion amongst patients with Atrial fibrillation (AF) is fairly clear in contemporary clinical practice: AF with >48-hours duration either anticoagulate for minimum of 3 weeks or to perform imaging (Transoesophageal Echocardiography/Computed tomography of Chest) to rule out left atrium (LA) clot prior to cardioversion. In EMANATE trial many patients underwent cardioversion without imaging (34 patients with loaded apixaban dose, and 92 patients with 5 doses of non-loaded dose of apixaban). This is quite questionable in contemporary clinical practice, particularly as in your table 4, in approximately 45% patients LA clot persisted, despite anticoagulation around 39 days.
Response from Authors:
The use of anticoagulation in conjunction with imaging varied in the EMANATE trial as it does in contemporary clinical practice. To qualify for entry in the trial, patients could have no more than 48 hours of anticoagulation prior to randomization. Whether to obtain imaging for detection of thrombus in the left atrial appendage and in patients randomized to apixaban whether to administer a loading dose was determined by the local investigator caring for the patient. Among the 753 patients in the apixaban group, 418 underwent imaging, 342 received loading doses before cardioversion and 92 had a loading dose, but not imaging. None of these patients developed stroke or systemic embolism. The mean interval from randomization to cardioversion was 4.1 days. The safety of performing cardioversion without imaging after only brief anticoagulation, suggested by these observations, requires validation in a larger patient cohort.
Authors: Michael D. Ezekowitz, Charles V. Pollack Jr, Jonathan L. Halperin, Jonas Oldgren