-
PDF
- Split View
-
Views
-
Cite
Cite
Ahmed N Mahmoud, Mohamed M Gad, Akram Y Elgendy, Islam Y Elgendy, Anthony A Bavry, Efficacy and safety of aspirin for primary prevention of cardiovascular events: a meta-analysis and trial sequential analysis of randomized controlled trials, European Heart Journal, Volume 40, Issue 7, 14 February 2019, Pages 607–617, https://doi.org/10.1093/eurheartj/ehy813
Close - Share Icon Share
Abstract
The role of aspirin in the primary prevention setting is continuously evolving. Recent randomized trials have challenged the role of aspirin in the primary prevention setting.
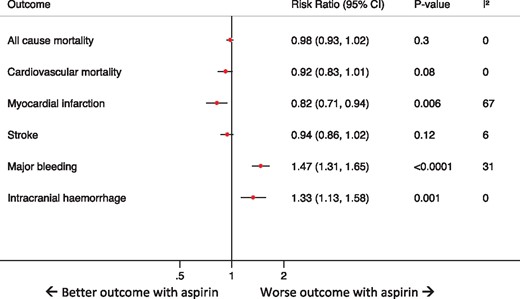
Electronic databases were searched for randomized trials that compared aspirin vs. placebo (or control) in subjects without established atherosclerotic disease. The primary efficacy outcome was all-cause mortality, while the primary safety outcome was major bleeding. Summary estimates were reported using a DerSimonian and Laird random effects model. A total of 11 trials with 157 248 subjects were included. At a mean follow-up of 6.6 years, aspirin was not associated with a lower incidence of all-cause mortality [risk ratio (RR) 0.98, 95% confidence interval (CI) 0.93–1.02; P = 0.30]; however, aspirin was associated with an increased incidence of major bleeding (RR 1.47, 95% CI 1.31–1.65; P < 0.0001) and intracranial haemorrhage (RR 1.33, 95% CI 1.13–1.58; P = 0.001). A similar effect on all-cause mortality and major bleeding was demonstrated in diabetic and high cardiovascular risk patients (i.e. 10-year risk >7.5%). Aspirin was associated with a lower incidence of myocardial infarction (RR 0.82, 95% CI 0.71–0.94; P = 0.006); however, this outcome was characterized by considerable heterogeneity (I 2 = 67%), and this effect was no longer evident upon limiting the analysis to the more recent trials. Trial sequential analysis confirmed the lack of benefit of aspirin for all-cause mortality up to a relative risk reduction of 5%.
Among adults without established cardiovascular disease, aspirin was not associated with a reduction in the incidence of all-cause mortality; however, it was associated with an increased incidence of major bleeding. The routine use of aspirin for primary prevention needs to be reconsidered.
Introduction
The use of aspirin for primary prevention of cardiovascular events in patients without prior history of atherosclerotic cardiovascular disease is a heavily debated topic, with a few guidelines recommending against its use,1 and others endorsing it in high-risk patients such as those with high cardiovascular risk2 or diabetics.3 The recommendation for aspirin use in primary prevention was largely based on a pooled analysis of six randomized trials that showed a reduction in ischaemic events with aspirin.4 With these conflicting recommendations, aspirin is still widely used among healthy individuals without established atherosclerosis in the hope of preventing myocardial infarction (MI) and death. For example, 21.8% reported taking aspirin for primary prevention, with only a slight decline from previous years in the United States.5 While the benefit of aspirin for patients with history of an acute ischaemic event (i.e. MI or ischaemic stroke) is better established, uncertainty remains regarding whether there is a favourable balance of benefit to harm for aspirin in the setting of primary prevention.6 , 7 A previous meta-analysis showed that aspirin reduced all-cause mortality, MI, and ischaemic stroke, with an increased risk of major bleeding,8 while a more recent meta-analysis concluded that aspirin reduced non-fatal MI, with little or no effect on cardiovascular or all-cause mortality, compared with control in primary prevention9; however, these analyses included several trials of patients with known atherosclerosis and peripheral vascular disease.10 , 11 This heterogeneity in the patient population selection might have impacted the findings of these meta-analyses. Moreover, the benefit of aspirin in these meta-analyses has been attributed to relatively few studies.6 , 7 Recently, the results of several large-scale randomized trials have been reported.12–14 Accordingly, a reappraisal of the current evidence based is warranted. Therefore, we aimed to perform an updated meta-analysis and trial sequential analysis of randomized trials to evaluate the efficacy and safety of aspirin among patients without prior known history of atherosclerotic cardiovascular disease.
Methods
Data sources
Major scientific databases including Pubmed, MEDLINE, Web of Science, and Embase were searched from inception till 25 September 2018 for randomized trials comparing aspirin with placebo or no aspirin control. The search was conducted without any language restrictions. The bibliographies of the included studies and prior meta-analyses on the same topic were also screened for trials not included by this search strategy. This meta-analysis was performed in concurrence with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Supplementary material online, Checkbox)S1 and was registered on PROSPERO international prospective register of systematic reviews (CRD42018111137).
Inclusion criteria
A study was included if it satisfied all of the following: (i) a randomized trial, (ii) comparing aspirin vs. placebo/no aspirin control, (iii) in adult patients without prior history of atherosclerosis (including peripheral arterial disease, coronary artery disease, prior MI, prior stroke or transient ischaemic attack, prior percutaneous coronary intervention, prior coronary artery bypass grafting), and (iv) including 500 patients or more. Trials that were conducted exclusively on diabetics were included in the final cohort, as long as they had no known history of atherosclerosis, with an intention to perform a sensitivity analysis for the primary outcomes by excluding these trials.
Data extraction
Two authors (A.N.M. and M.G.) performed the screening phase and the final extraction of the baseline characteristics of each trial (e.g. mean age, percentage of females, etc.), study quality (e.g. allocation concealment, methods of randomization, etc.), and outcomes of interest. A third author (A.Y.E.) then crosschecked the data for any errors during data extraction. Any disagreements were resolved by consensus among the authors.
Definition of outcomes
The primary efficacy outcome was all-cause mortality. We chose all-cause mortality as a primary outcome given its universal definition and it’s balance of efficacy and safety, resulting in the least heterogeneity among the studies. Secondary efficacy outcomes were cardiovascular mortality, fatal and non-fatal MI, and fatal and non-fatal ischaemic stroke. The primary safety outcome was major bleeding as defined by each trial. The secondary safety outcome was intracranial haemorrhage. All outcomes were defined as per the study’s definition.
Assessment of studies quality
As recommended by the Cochrane handbook of systematic reviews and meta-analysis, we performed the quality assessment of each study included using the Cochrane risk of bias tool. This consists of seven points that test for selection, performance, detection, attrition, and reporting biases.S2
Conventional meta-analysis statistical analysis
For descriptive purposes, weighted frequencies were calculated for categorical variables and weighted means and standard deviations (SD) were calculated for continuous variables, using the sample size of each trial as the weight. Meta-prop plug-in software of STATA® was used to calculate the random effects inverse-variance weighted incidences of each outcome of interest in the aspirin and placebo groups. Summary random effects risk ratios (RRs) were calculated for all outcomes using DerSimonian and Laird.S3 In order to account for the variation in follow-up between the included studies and to assess the impact of censoring, a secondary analysis was conducted for the primary outcomes of interest using hazard ratios (HRs) and 95% confidence intervals (CIs) (Supplementary material online, Methods). I 2 statistic was used to assess the heterogeneity between studies with values 0–30%, more than 30–60%, and more than 60% corresponding to low, moderate, and high degree of heterogeneity, respectively.S4 Publication bias for the primary efficacy outcome was assessed using Begg’s test.S5 Details of the subgroup analysis, sensitivity analysis, and meta-regressions conducted are reported in the Supplementary material online, Methods. A two-sided P-value of <0.05 and CI of 95% was considered to be statistically significant, and all statistical analysis for the conventional meta-analysis was performed with use of STATA® software version 14 (StataCorp, College Station, TX, USA).
Trial sequential analysis
A trial sequential analysis was conducted for the primary outcome of all-cause mortality, with calculation of the meta-analysis information size required to detect 10%, 7%, 6%, and 5% relative risk reduction of all-cause mortality in the aspirin group with power of 80% and alpha 5%. Further details are reported in the Supplementary material online, Methods.
Results
Included studies and participants’ baseline characteristics
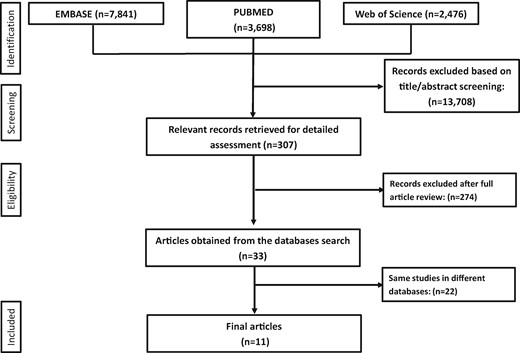
Among 14 015 records included in our initial search of Pudmed, Embase, and Web of Science, a total of 11 studies with 157 248 participants met our inclusion criteria12–22 (Figure 1, Supplementary material online, Figures S1–S3). The primary outcomes of the included studies were the composite of cardiovascular events (details of the definition of the each individual composite are summarized Supplementary material online, Table S1). Four trials compared aspirin with no aspirin control,15 , 16 , 20 , 22 two trials were conducted only on patients with diabetes,13 , 15 one trial included female health professionals,19 and two trials were conducted on male health professionals.18 , 22 Two trials used a daily dose of aspirin >100 mg/day.18 , 22 Two trials included approximately 5–6% of the patients with prior history of heart disease.17 , 22 Given the small proportion of these patients in each of the trials we decided to include them in the main analysis, and performed a sensitivity analysis for the primary outcomes by excluding them. We excluded three trials that were previously included in prior meta-analyses; two trials evaluated aspirin in patients with asymptomatic peripheral arterial disease10 , 11 and the third study included up to 49% of the patient with history of cardiovascular disease.23 This approach was chosen to minimize the heterogeneity among the final cohort. However, we performed a secondary analysis for all the outcomes by including these three trials.
Summary of how the systematic search was conducted and eligible studies were identified (PRISMA flow diagram). Pubmed, Embase and Web of Science search strategies are illustrated in Supplementary material online, Figures S1 –S3.
The overall weighted mean follow-up duration was 6.6 years (SD = 0.7 years). The mean age of the total population was 61.3 years (SD = 2.2 years). 14% of the participants were smokers and 52% were females. Details of the baseline characteristics of the individual studies are reported in Table 1. Two trials21 , 22 did not report major adverse cardiovascular events (MACE), and thus were not included in the subgroup analysis according to cardiovascular risk. Two15 , 18 studies had clear risks of bias per the Cochrane risk assessment tool and were deemed as high risk of bias studies. Details regarding the evaluation of the risk of bias are reported in the Supplementary material online, Table S2.
Study baseline characteristics
| Trial . | ARRIVE12 . | ASCEND13 . | ASPREE14 . | JPAD15 . | JPPP16 . | WHS19 . | PPP20 . | TPT21 . | HOT17 . | PHS18 . | BMD22 . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | 2018 | 2018 | 2018 | 2016 | 2014 | 2005 | 2001 | 1998 | 1998 | 1989 | 1988 |
| Patient population | Patients with moderate cardiovascular risk | Diabetic patients without known cardiovascular disease | People without cardiovascular disease, dementia, or disability | Diabetic patients | Patients with hypertension, dyslipidaemia, or diabetes | Female health professionals | Patients over 50 years | Men at increased cardiovascular risk | Hypertensive patients | Male physicians | Male physicians |
| Control arm | Placebo | Placebo | Placebo | No ASA | No ASA | Placebo | No ASA | Placebo | Placebo | Placebo | No ASA |
| Follow-up (years) | 5 | 7.4 | 4.7 | 10.3 | 6.5 | 10.1 | 3.6 | 6.8 | 3.8 | 5 | 6 |
| Lost to follow-up (%) | 3/3 | 1/1 | 2/2 | 38/34 | 10/11 | 1/3 | 1/1 | — | 3a | — | 0/0 |
| 10-Year MACEb in control arm (%) | 5.7 | 10.8 | 7.8 | 12.9 | 5.9 | 2.6 | 7.8 | — | 10.5 | 6.7 | — |
| Enrolment range (mid enrolment year) | 2007–2016 (2012) | 2005–2011 (2008) | 2010–2014 (2012) | 2003–2005 (2005) | 2005–2007 (2006) | 1992–1995 (1993) | 1994–1998 (1996) | 1984–1989 (1987) | 1992–1996 (1994) | 1981–1987 (1984) | 1978–1979 (1978) |
| Risk of bias | Low | Low | Low | High | Low | Low | Low | Low | Low | High | Low |
| Number of patients | 6270/6276 | 7740/7740 | 9525/ 9589 | 1262/1277 | 7323/7335 | 19 934/ 19 942 | 2226/2269 | 1268/1272 | 9399/9391 | 11 037/ 11 034 | 3429/1710 |
| Mean age (years) | 63.9/63.9 | 63.2/63.3 | 50% ≥74 years | 65/64 | 70.6/70.5 | 54.6/54.6 | 64.4/64.4 | 57.7/57.3 | 61.5/61.5 | 55% >50 years | 53% ≥60 years |
| Females (%) | 30/30 | 37/38 | 56/56 | 43/46 | 58/58 | 100/100 | 57/58 | 0/0 | 47/47 | 0/0 | 0/0 |
| Hypertension (%) | 63/63 | 62/62 | 74/75 | 59/57 | 85/85 | 26/26 | 69/68 | 139/139c | 170/170c | 39/39 | 9/10 |
| Smoking (%) | 29/29 | 8/8 | 4/4 | 23/19 | 13/13 | 13/13 | 15/15 | 41/42 | 16/16 | 11/11 | 31/32 |
| Daily dose of aspirin (mg/day) | 100 | 100 | 100 | 81/100 | 100 | 100d | 100 | 75 | 75 | 325d | 500 (or 300) |
| Trial . | ARRIVE12 . | ASCEND13 . | ASPREE14 . | JPAD15 . | JPPP16 . | WHS19 . | PPP20 . | TPT21 . | HOT17 . | PHS18 . | BMD22 . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | 2018 | 2018 | 2018 | 2016 | 2014 | 2005 | 2001 | 1998 | 1998 | 1989 | 1988 |
| Patient population | Patients with moderate cardiovascular risk | Diabetic patients without known cardiovascular disease | People without cardiovascular disease, dementia, or disability | Diabetic patients | Patients with hypertension, dyslipidaemia, or diabetes | Female health professionals | Patients over 50 years | Men at increased cardiovascular risk | Hypertensive patients | Male physicians | Male physicians |
| Control arm | Placebo | Placebo | Placebo | No ASA | No ASA | Placebo | No ASA | Placebo | Placebo | Placebo | No ASA |
| Follow-up (years) | 5 | 7.4 | 4.7 | 10.3 | 6.5 | 10.1 | 3.6 | 6.8 | 3.8 | 5 | 6 |
| Lost to follow-up (%) | 3/3 | 1/1 | 2/2 | 38/34 | 10/11 | 1/3 | 1/1 | — | 3a | — | 0/0 |
| 10-Year MACEb in control arm (%) | 5.7 | 10.8 | 7.8 | 12.9 | 5.9 | 2.6 | 7.8 | — | 10.5 | 6.7 | — |
| Enrolment range (mid enrolment year) | 2007–2016 (2012) | 2005–2011 (2008) | 2010–2014 (2012) | 2003–2005 (2005) | 2005–2007 (2006) | 1992–1995 (1993) | 1994–1998 (1996) | 1984–1989 (1987) | 1992–1996 (1994) | 1981–1987 (1984) | 1978–1979 (1978) |
| Risk of bias | Low | Low | Low | High | Low | Low | Low | Low | Low | High | Low |
| Number of patients | 6270/6276 | 7740/7740 | 9525/ 9589 | 1262/1277 | 7323/7335 | 19 934/ 19 942 | 2226/2269 | 1268/1272 | 9399/9391 | 11 037/ 11 034 | 3429/1710 |
| Mean age (years) | 63.9/63.9 | 63.2/63.3 | 50% ≥74 years | 65/64 | 70.6/70.5 | 54.6/54.6 | 64.4/64.4 | 57.7/57.3 | 61.5/61.5 | 55% >50 years | 53% ≥60 years |
| Females (%) | 30/30 | 37/38 | 56/56 | 43/46 | 58/58 | 100/100 | 57/58 | 0/0 | 47/47 | 0/0 | 0/0 |
| Hypertension (%) | 63/63 | 62/62 | 74/75 | 59/57 | 85/85 | 26/26 | 69/68 | 139/139c | 170/170c | 39/39 | 9/10 |
| Smoking (%) | 29/29 | 8/8 | 4/4 | 23/19 | 13/13 | 13/13 | 15/15 | 41/42 | 16/16 | 11/11 | 31/32 |
| Daily dose of aspirin (mg/day) | 100 | 100 | 100 | 81/100 | 100 | 100d | 100 | 75 | 75 | 325d | 500 (or 300) |
Data are presented as aspirin/control and percentages are approximated to the nearest integer.
MACE, major adverse cardiovascular events.
Percentage is of the total cohort.
MACE was defined as composite of cardiovascular death, non-fatal myocardial infarction and non-fatal stroke, whenever possible.
Mean systolic blood pressure in both arms.
Every other day doses.
Study baseline characteristics
| Trial . | ARRIVE12 . | ASCEND13 . | ASPREE14 . | JPAD15 . | JPPP16 . | WHS19 . | PPP20 . | TPT21 . | HOT17 . | PHS18 . | BMD22 . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | 2018 | 2018 | 2018 | 2016 | 2014 | 2005 | 2001 | 1998 | 1998 | 1989 | 1988 |
| Patient population | Patients with moderate cardiovascular risk | Diabetic patients without known cardiovascular disease | People without cardiovascular disease, dementia, or disability | Diabetic patients | Patients with hypertension, dyslipidaemia, or diabetes | Female health professionals | Patients over 50 years | Men at increased cardiovascular risk | Hypertensive patients | Male physicians | Male physicians |
| Control arm | Placebo | Placebo | Placebo | No ASA | No ASA | Placebo | No ASA | Placebo | Placebo | Placebo | No ASA |
| Follow-up (years) | 5 | 7.4 | 4.7 | 10.3 | 6.5 | 10.1 | 3.6 | 6.8 | 3.8 | 5 | 6 |
| Lost to follow-up (%) | 3/3 | 1/1 | 2/2 | 38/34 | 10/11 | 1/3 | 1/1 | — | 3a | — | 0/0 |
| 10-Year MACEb in control arm (%) | 5.7 | 10.8 | 7.8 | 12.9 | 5.9 | 2.6 | 7.8 | — | 10.5 | 6.7 | — |
| Enrolment range (mid enrolment year) | 2007–2016 (2012) | 2005–2011 (2008) | 2010–2014 (2012) | 2003–2005 (2005) | 2005–2007 (2006) | 1992–1995 (1993) | 1994–1998 (1996) | 1984–1989 (1987) | 1992–1996 (1994) | 1981–1987 (1984) | 1978–1979 (1978) |
| Risk of bias | Low | Low | Low | High | Low | Low | Low | Low | Low | High | Low |
| Number of patients | 6270/6276 | 7740/7740 | 9525/ 9589 | 1262/1277 | 7323/7335 | 19 934/ 19 942 | 2226/2269 | 1268/1272 | 9399/9391 | 11 037/ 11 034 | 3429/1710 |
| Mean age (years) | 63.9/63.9 | 63.2/63.3 | 50% ≥74 years | 65/64 | 70.6/70.5 | 54.6/54.6 | 64.4/64.4 | 57.7/57.3 | 61.5/61.5 | 55% >50 years | 53% ≥60 years |
| Females (%) | 30/30 | 37/38 | 56/56 | 43/46 | 58/58 | 100/100 | 57/58 | 0/0 | 47/47 | 0/0 | 0/0 |
| Hypertension (%) | 63/63 | 62/62 | 74/75 | 59/57 | 85/85 | 26/26 | 69/68 | 139/139c | 170/170c | 39/39 | 9/10 |
| Smoking (%) | 29/29 | 8/8 | 4/4 | 23/19 | 13/13 | 13/13 | 15/15 | 41/42 | 16/16 | 11/11 | 31/32 |
| Daily dose of aspirin (mg/day) | 100 | 100 | 100 | 81/100 | 100 | 100d | 100 | 75 | 75 | 325d | 500 (or 300) |
| Trial . | ARRIVE12 . | ASCEND13 . | ASPREE14 . | JPAD15 . | JPPP16 . | WHS19 . | PPP20 . | TPT21 . | HOT17 . | PHS18 . | BMD22 . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | 2018 | 2018 | 2018 | 2016 | 2014 | 2005 | 2001 | 1998 | 1998 | 1989 | 1988 |
| Patient population | Patients with moderate cardiovascular risk | Diabetic patients without known cardiovascular disease | People without cardiovascular disease, dementia, or disability | Diabetic patients | Patients with hypertension, dyslipidaemia, or diabetes | Female health professionals | Patients over 50 years | Men at increased cardiovascular risk | Hypertensive patients | Male physicians | Male physicians |
| Control arm | Placebo | Placebo | Placebo | No ASA | No ASA | Placebo | No ASA | Placebo | Placebo | Placebo | No ASA |
| Follow-up (years) | 5 | 7.4 | 4.7 | 10.3 | 6.5 | 10.1 | 3.6 | 6.8 | 3.8 | 5 | 6 |
| Lost to follow-up (%) | 3/3 | 1/1 | 2/2 | 38/34 | 10/11 | 1/3 | 1/1 | — | 3a | — | 0/0 |
| 10-Year MACEb in control arm (%) | 5.7 | 10.8 | 7.8 | 12.9 | 5.9 | 2.6 | 7.8 | — | 10.5 | 6.7 | — |
| Enrolment range (mid enrolment year) | 2007–2016 (2012) | 2005–2011 (2008) | 2010–2014 (2012) | 2003–2005 (2005) | 2005–2007 (2006) | 1992–1995 (1993) | 1994–1998 (1996) | 1984–1989 (1987) | 1992–1996 (1994) | 1981–1987 (1984) | 1978–1979 (1978) |
| Risk of bias | Low | Low | Low | High | Low | Low | Low | Low | Low | High | Low |
| Number of patients | 6270/6276 | 7740/7740 | 9525/ 9589 | 1262/1277 | 7323/7335 | 19 934/ 19 942 | 2226/2269 | 1268/1272 | 9399/9391 | 11 037/ 11 034 | 3429/1710 |
| Mean age (years) | 63.9/63.9 | 63.2/63.3 | 50% ≥74 years | 65/64 | 70.6/70.5 | 54.6/54.6 | 64.4/64.4 | 57.7/57.3 | 61.5/61.5 | 55% >50 years | 53% ≥60 years |
| Females (%) | 30/30 | 37/38 | 56/56 | 43/46 | 58/58 | 100/100 | 57/58 | 0/0 | 47/47 | 0/0 | 0/0 |
| Hypertension (%) | 63/63 | 62/62 | 74/75 | 59/57 | 85/85 | 26/26 | 69/68 | 139/139c | 170/170c | 39/39 | 9/10 |
| Smoking (%) | 29/29 | 8/8 | 4/4 | 23/19 | 13/13 | 13/13 | 15/15 | 41/42 | 16/16 | 11/11 | 31/32 |
| Daily dose of aspirin (mg/day) | 100 | 100 | 100 | 81/100 | 100 | 100d | 100 | 75 | 75 | 325d | 500 (or 300) |
Data are presented as aspirin/control and percentages are approximated to the nearest integer.
MACE, major adverse cardiovascular events.
Percentage is of the total cohort.
MACE was defined as composite of cardiovascular death, non-fatal myocardial infarction and non-fatal stroke, whenever possible.
Mean systolic blood pressure in both arms.
Every other day doses.
Efficacy outcomes
All-cause mortality
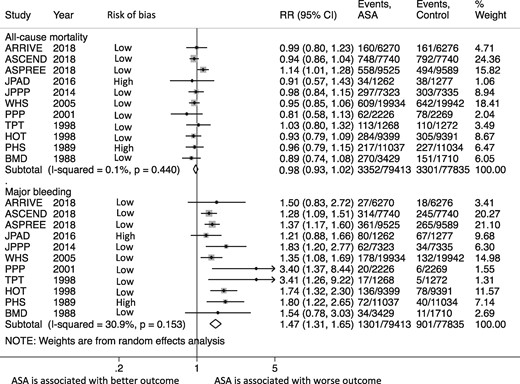
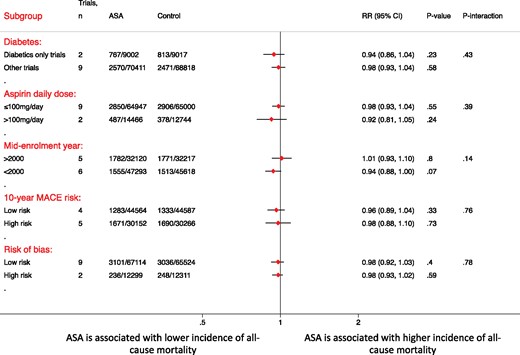
The incidence of all-cause mortality was similar between the aspirin and control groups [4.6% (95% CI 3.4–5.8%) vs. 4.7% (95% CI 3.6–5.9%); RR = 0.98, 95% CI 0.93–1.02, P = 0.30, I 2 = 0%] (Figure 2). The secondary analysis using HRs showed similar results (HR 0.98, 95% CI 0.93–1.04, P = 0.51, I 2 = 17%). This effect was consistent after exclusion of non-placebo controlled trials (RR = 0.99, 95% CI 0.93–1.05, P = 0.68, I 2 = 22%), the two trials reporting a minority of patients with known cardiovascular disease (RR = 0.99, 95% CI 0.94–1.04, P = 0.63, I 2 = 6.6%), and upon excluding each trial at a time (Supplementary material online, Figure S4). Subgroup analyses according to the 10-years risk, diabetes, mid-enrolment year, aspirin dose, risk of bias, and follow-up duration showed similar mortality in all subgroups without any evidence of subgroup interaction (Figure 3 and Supplementary material online, Figure S5). Meta-regression by mid-enrolment year, mean age, percentage of females, hypertension (HTN), and smoking did not show any evidence of effect modification. (P = 0.74, 0.69, 0.45, 0.76, 0.58, respectively) (Supplementary material online, Figure S6). There was no evidence of publication bias by Begg’s test (P > 0.99).
Summary plot for primary efficacy (all-cause mortality) and safety (major bleeding) outcomes. The relative size of the data markers indicates the weight of the sample size from each study. ASA, aspirin; CI, confidence interval; RR, risk ratio.
A forest plot illustrating the risk ratio and 95% confidence intervals of all-cause mortality according to various subgroups of interest. ASA, aspirin; CI, confidence interval; RR, risk ratio.
Secondary efficacy outcomes
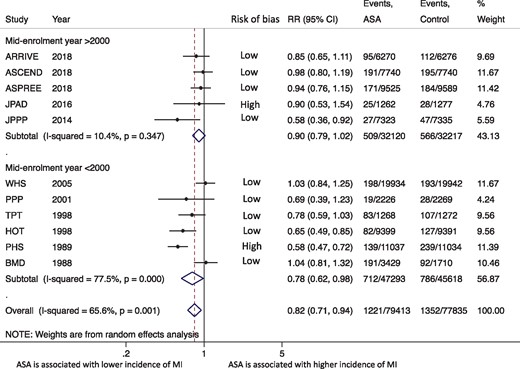
To allow for more homogeneity in the outcomes definitions, we included fatal and non-fatal events in the final analysis, whenever reported (i.e. fatal and non-fatal MI and stroke). The definition of cardiovascular mortality and MI are reported in the Supplementary material online,Tables S3 and S4. Silent MI was reported in one trial17 and two trials reported sudden cardiac death as a separate outcome.15 , 18 The incidence of cardiovascular mortlity [1.3% (95% CI 1.0–1.6%) vs. 1.4% (95% CI 1.1–1.7%); RR = 0.92, 95% CI 0.83–1.01, P = 0.08, I 2 = 0%] (Supplementary material online, Figure S7), and ischaemic stroke [1.7% (95% CI 1.3–2.0%) vs. 1.8% (95% CI 1.4–2.2%); RR = 0.94, 95% CI 0.86–1.02, P = 0.12, I 2 = 6%] (Supplementary material online, Figure S8) were similar between both groups. The incidence of MI was lower with aspirin [1.9% (95% CI 1.2–2.6%) vs. 2.2% (95% CI 1.6–3.1%); RR = 0.82, 95% CI 0.71–0.94, P = 0.006, I 2 = 67%] [number needed to treat (NNT) = 333]; however, the effect size was characterized by high degree of heterogeneity between the studies included (Supplementary material online, Figure S9). The risk of MI remained lower with aspirin even after inclusion of silent MI and sudden cardiac death events (Supplementary material online, Figure S10). A secondary analysis excluding older trials with mid-enrolment year prior to 2000 (i.e. trials predating the universal definitions of MI and using non-contemporary cardiac markers) showed the lack of aspirin benefit in more recent trials (RR = 0.90, 95% CI 0.79–1.02, P = 0.10, I 2 = 10%) (Figure 4).
A subgroup forest plot for myocardial infarction outcome according to the mid-enrolment year of the included trials. ASA, aspirin; CI, confidence interval; RR, risk ratio.
Safety outcomes
Major bleeding
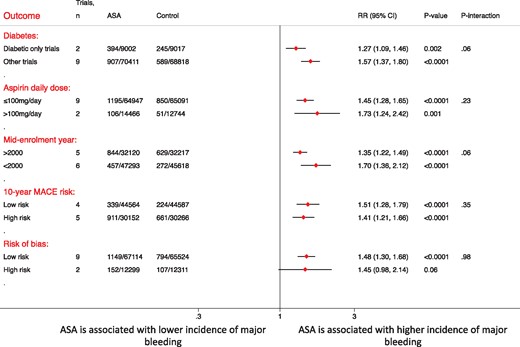
The definition of major bleeding as reported by each study is summarized in Supplementary material online, Table S5. The incidence of major bleeding was higher with aspirin [1.8% (95% CI 1.3–3.4%) vs. 1.2% (95% CI 0.8–1.6%); RR = 1.47, 95% CI 1.31–1.65, P < 0.0001, I 2 = 31%] [Number needed to harm (NNH) = 250] (Figure 2). A secondary analysis using the HRs showed similar findings (HR 1.47, 95% CI 1.31–1.64, P < 0.0001, I 2 = 30%). The results did not change after exclusion of the non-placebo controlled trials (RR = 1.44, 95% CI 1.28–1.62, P < 0.0001, I 2 = 29%) and trials reporting a minor percentage of patients with history of cardiovascular disease (RR = 1.44, 95% CI 1.27–1.63, P < 0.0001, I 2 = 34). The effect was consistent and upon excluding each trial at a time (Supplementary material online, Figure S11). Subgroup analysis did not show any difference in major bleeding risk between across the different subgroups (Figure 5 and Supplementary material online, Figure S5). Meta-regression by mid-enrolment year, mean age, females, HTN, and smoking did not show any evidence of effect modification (P = 0.15, 0.76, 0.41, 0.94, 0.34, respectively) (Supplementary material online, Figure S12).
A forest plot illustrating the risk ratio and 95% confidence intervals of major bleeding according to various subgroups of interest. ASA, aspirin; CI, confidence interval; RR, risk ratio.
Intracranial haemorrhage
The definition of intracranial haemorrhage per included study is reported in the Supplementary material online, Table S6. Aspirin was associated with higher risk of intracranial haemorrhage [0.4% (95% CI 0.2–0.5%) vs. 0.3% (95% CI 0.1–0.4%); RR = 1.33, 95% CI 1.13–1.58, P = 0.001, I 2 = 0%] (Supplementary material online, Figure S13).
A secondary analysis was conducted to assess the impact of excluding the Prevention of Progression of Arterial Disease and Diabetes (POPADAD), Aspirin for Asymptomatic Atherosclerosis (AAA), and Early Treatment Diabetic Retinopathy Study (ETDRS) trials.10 , 11 , 23 There was no significant difference in any of the outcomes by including these three trials in the secondary analysis (Supplementary material online, Table S7). Summary estimates for all outcomes of this meta-analysis are presented in the Take home figure.
A forest plot illustrating the risk ratios and 95% confidence interval for all outcomes of interest.
Trial sequential analysis
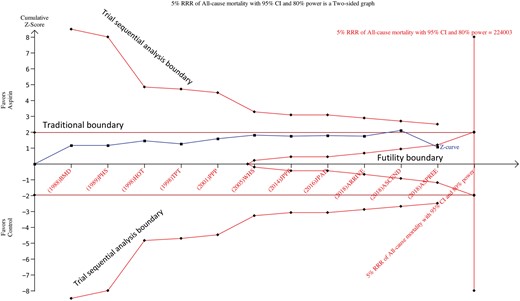
The required information sized for the outcome of all-cause mortality was calculated based on a proportion of 4.7% events in the control group. A relative risk reduction of 10% in the aspirin group was initially evaluated (corresponding to an absolute risk reduction of 0.47%) followed by 7% (absolute risk reduction of 0.33%) then finally 5% (absolute risk reduction of 0.24%) at an alpha of 5% and power of 80%. The cumulative z curve did not cross the trial sequential analysis boundary or the traditional significance boundary but crossed the futility boundary at 10% (Supplementary material online, Figure S14), 7% (Supplementary material online, Figure S15), and 5% relative risk reductions (Figure 6), supporting the findings of the conventional meta-analysis (i.e. lack of benefit from aspirin on all-cause mortality), and also confirming that future trials are not useful for further evaluation of this effect.
Trial sequential analysis of all-cause mortality using random effects meta-analysis, based on an anticipated intervention effect of 5% relative risk reduction, a control event incidence of 4.7%, and absence of diversity (0%), with alpha 5% and power 80%.
Discussion
This meta-analysis of 11 trials with >157 000 individuals without history of atherosclerotic disease demonstrated that aspirin was not associated with a reduction in the risk of all-cause mortality in the primary prevention setting. This was further confirmed by performing a trial sequential analysis, which also suggested the futility of conducting further trials to assess a benefit of aspirin on all-cause mortality. The lack of benefit was evident even in diabetics and patients with high cardiovascular risk (i.e. 10-year risk >7.5%). While aspirin was associated with a small absolute reduction in the risk of MI (i.e. 0.3%, NNT = 333), this outcome was characterized by considerable degree of heterogeneity, and this small benefit was lost upon limiting the analysis to the more contemporary trials (i.e. suggesting the lack of a robust association between aspirin use and reduction in the risk of MI). Aspirin was associated with an absolute increase in major bleeding of 0.6% (NNH = 250) and an absolute increase in intracranial haemorrhage of 0.1% (NNH = 1000). These results suggest that even if there is any evidence of only marginal benefit, this is offset by a modest harm. Collectively, these findings confirm the lack of overall benefit from routine aspirin use in the setting of primary prevention, and suggest a possible harm in the contemporary era.
Aspirin for primary prevention has been advocated by some physicians for prevention of ischaemic events, which has been extrapolated from secondary prevention trials.2 , 3 The argument for using aspirin in this setting relies on the prevention of thrombus propagation and plaque rupture. The counterargument for this theoretical benefit is that aspirin might induce haemorrhage in the plaque and thus precipitating an event.24 Interestingly, only three of the included randomized trials have shown a reduction in the risk of MI with aspirin.16–18 Notably, two of these trials were terminated early due to futility, and the primary endpoint, which was initially all-cause mortality, was later on changed in both trials.16 , 18 Despite a substantial reduction in the risk of MI in one of these trials,18 this benefit was not translated into a reduction in the risk of all-cause or cardiovascular mortality in that trial. In this meta-analysis, we noted the benefit of aspirin on reducing the risk of MI only in the earlier trials, when the efforts on modifying other risk factor such as blood pressure control, and smoking cessation were not largely emphasized.25 Another important consideration is that up to 50% of MIs are clinically silent26 and these silent MIs carry a similar long-term mortality to clinically recognized MI,27 therefore relying on this outcome to ascertain a benefit from long-term aspirin use might be challenging, and making a stronger case to assess all-cause mortality as an endpoint to assess for any benefit. Unlike statin therapy in the primary prevention setting where there is stronger evidence supporting its benefit with minimal risk,28 aspirin therapy appears to carry no benefit and is associated with potential harm. One argument might be to consider using aspirin for reducing the risk of cancer,29 again this evidence was based mainly on old trials and has not been replicated in the more recent trials.13 , 30 Further, this meta-analysis found no evidence of reduction in all-cause mortality, thus disputing any potential mortality benefit that could be driven from cancer incidence reduction at least at the short to mid follow-up duration.
Some guideline societies as the American Heart Association/American Diabetes Association endorse aspirin use for diabetics with intermediate risk (5–10% 10-year MACE risk) for primary prevention.3 Recently, two randomized trials have evaluated the benefit of aspirin in diabetics;13 , 15 the Japanese Primary Prevention of Atherosclerosis With Aspirin for Diabetes (JPAD) trial showed no benefit at 10-years but an increased risk of gastrointestinal bleeding,15 while the A Study of Cardiovascular Events in Diabetes (ASCEND) trial showed a 12% reduction in ischaemic events with aspirin but offsets with a 29% increased risk of major bleeding.13 Notably, the proportion of patients on statin therapy in the ASCEND trial was considerably higher than in those in the JPAD trial (75% in the ASCEND when compared with 25% in the JPAD), which might have contributed to the benefit which was seen in the ASCEND trial. Our results showed a lack of benefit regarding all-cause mortality among diabetics and those with high cardiovascular risk, and are in support of the recent European Society of Cardiology recommendations against aspirin use in patients with diabetes, who do not have any prior history of established cardiovascular disease.1 It is important to note that these findings are driven from a subgroup analysis and should be considered hypothesis generating, and patient-level analyses of these trials are encouraged to confirm these findings.
The results of our current meta-analysis are consistent with recent evidence assessing the impact of aspirin on all-cause mortality in primary prevention setting.28 The strengths of this analysis include the low degree of heterogeneity for most of the assessed outcomes (except for MI), as a direct result of the careful inclusion criteria to maintain a homogeneous patient population, as well as the large sample size which was adequate to assess rare outcomes such as all-cause mortality and cardiovascular mortality in a low-risk population as confirmed by the trial sequential analysis. Our analysis strictly followed both the Cochrane and PRIMSA recommendations for meta-analysis reporting. We also performed a trial sequential analysis to confirm the lack of all-cause mortality benefit seen by conventional meta-analysis and indicated the futility for further trials to evaluate that outcome. Despite these strengths, this analysis is not without limitations. First, there is considerable variation in the definition of MI that might have contributed to the high level of heterogeneity seen in the effect size. We attempted to overcome such limitation by including the most consistent outcome definitions reported in all trials and by conducting various sensitivity analyses to explore the reasons for such heterogeneity. Second, there was a variable degree of follow-up, thus we performed an analysis comparing trials with <5 years vs. >5 years follow-up. Third, there was some degree of loss of follow-up reported among some of the trials, hence we conducted our analysis as intention to treat rather than as-treated and conducted a secondary analysis for the primary outcomes according HRs in an attempt to minimize such effect. Fourth, we could not comment on the effect of concomitant use of proton pump inhibitors on major bleeding since these data are not reported in most of the trials. Fifth, the control group in some of the included trials was a non-placebo comparator, which implies that these trials were not blinded. Therefore, we performed a sensitivity analysis for the primary outcomes by excluding non-placebo controlled trials. Finally, due to lack of patient-level data, we could not comment on the certain patient subsets that might benefit from low-dose aspirin for primary prevention. However, we performed multiple subgroup and meta-regression analyses for various factors and did not identify any potential subgroup or baseline characteristic, which might benefit from aspirin.
Conclusion
Aspirin use among healthy individuals without known atherosclerosis appears to be associated with increased harm and lack of mortality benefit. In this setting, aspirin is possibly associated with a modest reduction in MI risk; however, this comes at a cost of increased major bleeding and including intracranial haemorrhage. The routine use of aspirin for primary prevention needs to be reconsidered.
Conflict of interest: A.A.B. discloses the following relationship—Honorarium from American College of Cardiology and Edwards Lifesciences. All authors report that they have no involvements that might raise the question of bias in the work reported or in the conclusions, implications, or opinions stated.
Footnotes
See page 618 for the editorial comment on this article (doi: 10.1093/eurheartj/ehy872)
References
Antithrombotic Trialists' (ATT) Collaboration
Steering Committee of the Physicians' Health Study Research Group.
Author notes
Ahmed N. Mahmoud and Islam Y. Elgendy authors contributed equally to this study.