-
PDF
- Split View
-
Views
-
Cite
Cite
Hee Tae Yu, Jaemin Shim, Junbeom Park, Tae-Hoon Kim, Jae-Sun Uhm, Jong-Youn Kim, Boyoung Joung, Moon-Hyoung Lee, Young-Hoon Kim, Hui-Nam Pak, When is it appropriate to stop non-vitamin K antagonist oral anticoagulants before catheter ablation of atrial fibrillation? A multicentre prospective randomized study, European Heart Journal, Volume 40, Issue 19, 14 May 2019, Pages 1531–1537, https://doi.org/10.1093/eurheartj/ehy870
Close - Share Icon Share
Abstract
Although a recent expert consensus statement has recommended periprocedural uninterrupted (UI) non-vitamin K antagonist oral anticoagulants (NOACs) during catheter ablation of atrial fibrillation (AF) as a Class I indication, there have been no clear randomized trials. We investigated the safety and efficacy of UI, procedure day single-dose skipped (SDS), and 24-hour skipped (24S) NOACs in patients undergoing AF ablation.
In this prospective, open-label, randomized multicentre trial, 326 patients (75% male, 58 ± 11 years old) scheduled for AF catheter ablation were randomly assigned in a 1:1:1 ratio to UI, SDS, and 24S at three tertiary hospitals. Bridging with low molecular weight heparin was carried out in the patients with persistent AF who were assigned to the 24S group. Dabigatran, rivaroxaban, and apixaban were assigned in order after randomization. The primary endpoint was the incidence of bleeding events within 1 month after ablation. The secondary endpoints included thrombo-embolic and other procedure-related complications. The intra-procedural heparin requirement was higher in the 24S group than others (P < 0.001), and the mean activated clotting time was comparable among the groups (P = 0.139). The incidence of major bleeding up to 1 month after ablation and a post-procedural reduction in the haemoglobin levels did not significantly differ among the treatment groups and different NOACs (P > 0.05). There were no fatal events or thrombo-embolic complications in all the three groups.
In patients undergoing AF ablation, UI NOACs and SDS or double dose skipped NOACs had a comparable efficacy and safety, regardless of the type of NOAC.
See page 1538 for the editorial comment on this article (doi: 10.1093/eurheartj/ehz017)
Introduction
Radiofrequency catheter ablation (RFCA) is increasingly performed for patients with paroxysmal and persistent atrial fibrillation (AF).1 , 2 AF ablation carries a significant risk of thromboembolisms due to thrombus formation in the left atrium (LA) and intra-procedural conversion from AF to sinus rhythm.3 Various techniques, such as transoesophageal echocardiography (TOE), intra-cardiac echocardiography, and irrigated tip ablation catheters, have been used to reduce the risk of periprocedural thrombo-embolic complications, and the guidelines recommend heavy anticoagulation with an ACT of 300–400 s.4 In the warfarin era, an uninterrupted (UI) anticoagulation strategy without a traditional heparin bridge was recognized as the optimal treatment by several clinical studies.5 However, strategies for the use of non-vitamin K antagonist oral anticoagulants (NOACs) in the setting of AF ablation have varied, which may be associated with an increased incidence of thromboembolisms.6 The EHRA 2013 practice guidelines7 recommended that an NOAC should be stopped for longer than 48 h before high risk bleeding procedures, including AF ablation. The 2014 ACC/AHA/HRS guidelines8 generally advised that patients skip 1 or 2 doses of NOACs before catheter ablation of AF in the absence of antidotes. After several randomized clinical trials (RCT), such as the VENTURE AF9 and RE-CIRCUIT trials,10 in which UI rivaroxaban and dabigatran showed a non-inferior or superior outcome compared to UI warfarin within 1 month of the AF ablation, the 2017 HRS/EHRA/ECAS/APHRS/SOLACE expert consensus statement recommends UI dabigatran or rivaroxaban as a Class I indication.4 However, an antidote is not commercially available for NOACs (except for dabigatran), and the existing published data are limited from experienced institutions. Furthermore, since no direct RCT comparing the time of discontinuation of the NOAC has been conducted, it is still premature to generalize UI NOACs during the periprocedural period of AF catheter ablation. Particularly, periprocedural anticoagulation issues are more important in Asians, who have higher bleeding and stroke risks than other races.11 We previously compared UI warfarin and 24-hour skipped (24S) NOACs as a retrospective study, and there was no statistical difference in the major complications, but there were two minor strokes in the NOAC group.12 The purpose of this study was to compare the periprocedural complications in the 24S, same day single-dose skipped (SDS), and UI NOACs prior to the AF ablation procedure in a multicentre prospective RCT manner.
Methods
Study design and population
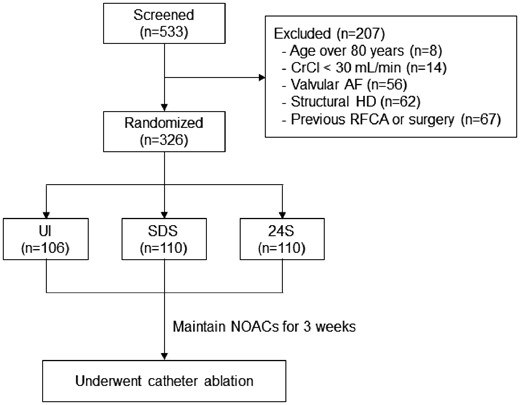
This trial was a randomized, open-label, multicentre trial, involving patients with non-valvular AF undergoing RFCA (ClinicalTrials.gov: NCT02504177). The study protocol adhered to the Declaration of Helsinki and was approved by the institutional review board at each participating centre. Proper written informed consent was obtained from all patients. The study cohort included 326 patients (75% male, 58 ± 11 years old) who underwent RFCA for symptomatic and drug-refractory non-valvular AF at three tertiary hospitals in Korea. Key exclusion criteria were as follows: (i) patients younger than 20 or older than 80 years; (ii) valvular AF; (iii) significant structural heart disease other than left ventricular hypertrophy; (iv) a left atrial (LA) diameter of 60 mm or greater; (v) creatinine clearance less than 30 mL/min; and (vi) a history of previous AF ablation or cardiac surgery. The anatomy of the LA and pulmonary veins (PVs) was visually defined by 3D computed tomography (CT) scans (64 Channel, Light Speed Volume CT, Philips, Brilliance 63, Amsterdam, Netherlands). All anti-arrhythmic drugs (AADs) were discontinued for a period of at least five half-lives.
Anticoagulation
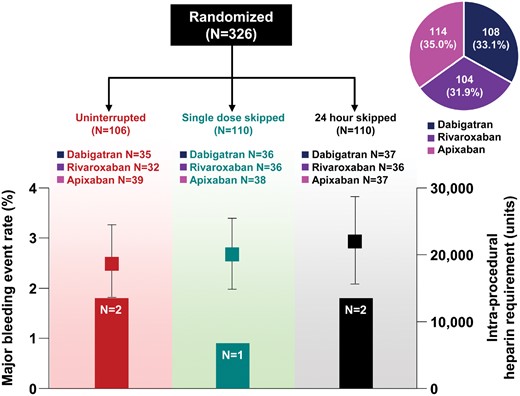
The patients were randomly assigned in a 1:1:1 ratio to the 24S, same day SDS, and UI NOAC groups (Figure 1). Dabigatran (n = 108), rivaroxaban (n = 104), and apixaban (n = 114) were assigned in order after randomization. Transoesophageal echocardiography or intra-cardiac echocardiography confirmed the absence of an LA thrombus before all ablation procedures. Preprocedural anticoagulation was maintained for at least 3 weeks before the RFCA. In patients with persistent AF with a CHA2DS2-VASc score of ≥2 who were assigned to the 24S group, we used low molecular weight heparin (enoxaparin 1 mg/kg) in the evening the day before the procedure. The activated clotting time (ACT) was kept at 350–400 s during the ablation procedure. Patients resumed anticoagulation at their usual dose on the evening of the procedure day if no prohibitive complications occurred. Anticoagulation was continued for at least 8 weeks after the procedure.
Patient enrollment and randomization. 24S, 24-hour skipped; AF, atrial fibrillation; CrCl, creatinine clearance; HD, heart disease; NOACs, non-vitamin K antagonist oral anticoagulants; RFCA, radiofrequency catheter ablation; SDS, single-dose skip; UI, uninterrupted.
Radiofrequency catheter ablation
An open-irrigated, 3.5-mm tip deflectable catheter (Celsius or SF56, Johnson & Johnson Inc., Diamond Bar, CA, USA; Coolflex or Flexibility, Abbott Inc., Minnetonka, MN, USA; 30–35 W; 45°C) was used for the RFCA. All patients initially underwent a circumferential PV isolation and creation of cavo-tricuspid isthmus block. For patients with persistent AF, a roof line, posterior inferior line, and anterior line were added as a standard lesion set. Depending on the operator’s discretion, additional ablation of the superior vena cava, non-PV foci, and complex fractionated electrograms was conducted. Non-PV foci under an isoproterenol infusion were also ablated. The procedure ended when there was no immediate recurrence of AF within 10 min after the cardioversion with an isoproterenol infusion (5–10 μg/min).
Study endpoints
The primary endpoint was the incidence of major bleeding events as defined by the International Society on Thrombosis and Hemostasis (ISTH).13 Major bleeding events were considered from the first femoral puncture to 1 month after the RFCA. The secondary endpoints included thrombo-embolic and other procedure-related events, such as vascular complications or minor bleeding events. Minor bleeding events were defined as clinical bleeding events that did not fulfil the ISTH criteria for major bleeding events. All outcome events were adjudicated by an independent committee in a blinded manner.
Statistical analysis
The current study was exploratory because a sample size large enough to provide a sufficient power to establish a formal non-inferiority trial would have made the trial unfeasible. Under the assumption that there was no difference in the absolute rates of major bleeding events between the three treatment groups, we decided that a descriptive comparison using ∼110 patients per group would generate clinically meaningful information. A central randomization strategy using computer-generated random permutation sequences was conducted to avoid any potential bias. Continuous variables were summarized as the mean ± standard deviation and were compared by a Student’s t-test and ANOVA. Categorical variables were summarized as a percentage of the group total and were compared by χ2 tests or Fisher’s exact tests. A two-sided P-value of less than 0.05 was considered to indicate statistical significance. The statistical analyses were performed using SPSS (version 23.0, Statistical Package for Social Sciences, Chicago, IL, USA) software for Windows.
Results
Baseline characteristics and intra-procedural variables
The baseline characteristics, including the comorbidities and echocardiographic parameters, were well balanced among the treatment groups (Table 1). The mean age was 58.3 ± 11.3 years. The majority of patients were male (74.5%) and had paroxysmal AF (62.0%). The mean CHA2DS2-VASc score was 1.7 ± 1.5, and 12.3% of the patients had a history of a previous stroke or transient ischaemic attack.
Baseline characteristics
| . | Total (n = 326) . | UI (n = 106) . | SDS (n = 110) . | 24S (n = 110) . |
|---|---|---|---|---|
| Age (years) | 58.3 ± 11.3 | 58.6 ± 11.7 | 57.9 ± 11.1 | 58.4 ± 11.3 |
| Male, n (%) | 243 (74.5) | 81 (76.4) | 79 (71.8) | 83 (75.5) |
| Paroxysmal AF, n (%) | 202 (62.0) | 67 (63.2) | 74 (67.3) | 61 (55.5) |
| AF duration (months) | 47.2 ± 51.7 | 47.3 ± 49.8 | 53.1 ± 58.3 | 41.3 ± 45.9 |
| BMI (kg/m2) | 25.3 ± 3.4 | 26.1 ± 3.7 | 24.7 ± 3.1 | 25.3 ± 3.3 |
| Body weight (kg) | 73.6 ± 18.4 | 73.9 ± 15.5 | 71.3 ± 17.8 | 75.6 ± 21.2 |
| CrCl (mL/min) | 93.2 ± 41.8 | 97.5 ± 54.1 | 90.9 ± 31.5 | 91.4 ± 37.0 |
| NOAC type | ||||
| Dabigatran, n (%) | 108 (33.1) | 35 (33.0) | 36 (32.7) | 37 (33.6) |
| Rivaroxaban, n (%) | 104 (31.9) | 32 (30.2) | 36 (32.7) | 36 (32.7) |
| Apixaban, n (%) | 114 (35.0) | 39 (36.8) | 38 (34.5) | 37 (33.6) |
| NOAC dosing | ||||
| Underdosinga, n (%) | 56 (17.2) | 21 (19.8) | 16 (14.5) | 19 (17.3) |
| Labelled use, n (%) | 269 (82.5) | 85 (80.2) | 93 (84.5) | 91 (82.7) |
| Overdosingb, n (%) | 1 (0.3) | 0 | 1 (0.9) | 0 |
| Comorbidities | ||||
| Heart failure, n (%) | 43 (13.2) | 11 (10.4) | 16 (14.5) | 16 (14.5) |
| Hypertension, n (%) | 140 (42.9) | 45 (42.5) | 45 (40.9) | 50 (45.5) |
| Diabetes, n (%) | 47 (14.4) | 10 (9.4) | 16 (14.5) | 21 (19.1) |
| Stroke/TIA, n (%) | 40 (12.3) | 12 (11.3) | 17 (15.5) | 11 (10.0) |
| Vascular disease, n (%) | 25 (7.7) | 8 (7.6) | 11 (10.0) | 6 (5.5) |
| CHA2DS2-VASc score | 1.7 ± 1.5 | 1.6 ± 1.4 | 1.7 ± 1.5 | 1.7 ± 1.4 |
| Echocardiographic measures | ||||
| LA dimension (mm) | 41.2 ± 6.7 | 41.0 ± 7.6 | 41.0 ± 6.0 | 41.6 ± 6.6 |
| LA volume index (mL/m2) | 39.1 ± 14.7 | 39.4 ± 13.2 | 39.2 ± 16.3 | 38.8 ± 14.6 |
| LVEDD (mm) | 49.8 ± 4.6 | 49.8 ± 4.5 | 49.3 ± 4.5 | 50.3 ± 4.8 |
| LV ejection fraction (%) | 61.3 ± 9.5 | 61.2 ± 9.0 | 61.3 ± 10.3 | 61.3 ± 9.5 |
| E/Em | 9.8 ± 4.0 | 9.7 ± 4.3 | 10.1 ± 4.2 | 9.5 ± 3.5 |
| . | Total (n = 326) . | UI (n = 106) . | SDS (n = 110) . | 24S (n = 110) . |
|---|---|---|---|---|
| Age (years) | 58.3 ± 11.3 | 58.6 ± 11.7 | 57.9 ± 11.1 | 58.4 ± 11.3 |
| Male, n (%) | 243 (74.5) | 81 (76.4) | 79 (71.8) | 83 (75.5) |
| Paroxysmal AF, n (%) | 202 (62.0) | 67 (63.2) | 74 (67.3) | 61 (55.5) |
| AF duration (months) | 47.2 ± 51.7 | 47.3 ± 49.8 | 53.1 ± 58.3 | 41.3 ± 45.9 |
| BMI (kg/m2) | 25.3 ± 3.4 | 26.1 ± 3.7 | 24.7 ± 3.1 | 25.3 ± 3.3 |
| Body weight (kg) | 73.6 ± 18.4 | 73.9 ± 15.5 | 71.3 ± 17.8 | 75.6 ± 21.2 |
| CrCl (mL/min) | 93.2 ± 41.8 | 97.5 ± 54.1 | 90.9 ± 31.5 | 91.4 ± 37.0 |
| NOAC type | ||||
| Dabigatran, n (%) | 108 (33.1) | 35 (33.0) | 36 (32.7) | 37 (33.6) |
| Rivaroxaban, n (%) | 104 (31.9) | 32 (30.2) | 36 (32.7) | 36 (32.7) |
| Apixaban, n (%) | 114 (35.0) | 39 (36.8) | 38 (34.5) | 37 (33.6) |
| NOAC dosing | ||||
| Underdosinga, n (%) | 56 (17.2) | 21 (19.8) | 16 (14.5) | 19 (17.3) |
| Labelled use, n (%) | 269 (82.5) | 85 (80.2) | 93 (84.5) | 91 (82.7) |
| Overdosingb, n (%) | 1 (0.3) | 0 | 1 (0.9) | 0 |
| Comorbidities | ||||
| Heart failure, n (%) | 43 (13.2) | 11 (10.4) | 16 (14.5) | 16 (14.5) |
| Hypertension, n (%) | 140 (42.9) | 45 (42.5) | 45 (40.9) | 50 (45.5) |
| Diabetes, n (%) | 47 (14.4) | 10 (9.4) | 16 (14.5) | 21 (19.1) |
| Stroke/TIA, n (%) | 40 (12.3) | 12 (11.3) | 17 (15.5) | 11 (10.0) |
| Vascular disease, n (%) | 25 (7.7) | 8 (7.6) | 11 (10.0) | 6 (5.5) |
| CHA2DS2-VASc score | 1.7 ± 1.5 | 1.6 ± 1.4 | 1.7 ± 1.5 | 1.7 ± 1.4 |
| Echocardiographic measures | ||||
| LA dimension (mm) | 41.2 ± 6.7 | 41.0 ± 7.6 | 41.0 ± 6.0 | 41.6 ± 6.6 |
| LA volume index (mL/m2) | 39.1 ± 14.7 | 39.4 ± 13.2 | 39.2 ± 16.3 | 38.8 ± 14.6 |
| LVEDD (mm) | 49.8 ± 4.6 | 49.8 ± 4.5 | 49.3 ± 4.5 | 50.3 ± 4.8 |
| LV ejection fraction (%) | 61.3 ± 9.5 | 61.2 ± 9.0 | 61.3 ± 10.3 | 61.3 ± 9.5 |
| E/Em | 9.8 ± 4.0 | 9.7 ± 4.3 | 10.1 ± 4.2 | 9.5 ± 3.5 |
All variables were not significantly different between the three treatment groups (P > 0.05).
24S, 24-hour skipped; AF, atrial fibrillation; BMI, body mass index; CHA2DS2-VASc, congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, prior stroke, transient ischaemic attack, or thromboembolism, vascular disease, age 65–74 years, sex category (female); CrCl, creatinine clearance; E/Em, early mitral inflow velocity over the early diastolic mitral annular velocity; LA, left atrium; LV, left ventricle; LVEDD, left ventricular end-diastolic dimension; NOAC, non-vitamin K antagonist oral anticoagulants; SDS, single-dose skip; TIA, transient ischaemic attack; UI, uninterrupted.
Main reasons for underdosing were concomitant use of antiplatelets and the eGFR change.
The reasons for overdosing were a deranged renal function and reduced body weight.
Baseline characteristics
| . | Total (n = 326) . | UI (n = 106) . | SDS (n = 110) . | 24S (n = 110) . |
|---|---|---|---|---|
| Age (years) | 58.3 ± 11.3 | 58.6 ± 11.7 | 57.9 ± 11.1 | 58.4 ± 11.3 |
| Male, n (%) | 243 (74.5) | 81 (76.4) | 79 (71.8) | 83 (75.5) |
| Paroxysmal AF, n (%) | 202 (62.0) | 67 (63.2) | 74 (67.3) | 61 (55.5) |
| AF duration (months) | 47.2 ± 51.7 | 47.3 ± 49.8 | 53.1 ± 58.3 | 41.3 ± 45.9 |
| BMI (kg/m2) | 25.3 ± 3.4 | 26.1 ± 3.7 | 24.7 ± 3.1 | 25.3 ± 3.3 |
| Body weight (kg) | 73.6 ± 18.4 | 73.9 ± 15.5 | 71.3 ± 17.8 | 75.6 ± 21.2 |
| CrCl (mL/min) | 93.2 ± 41.8 | 97.5 ± 54.1 | 90.9 ± 31.5 | 91.4 ± 37.0 |
| NOAC type | ||||
| Dabigatran, n (%) | 108 (33.1) | 35 (33.0) | 36 (32.7) | 37 (33.6) |
| Rivaroxaban, n (%) | 104 (31.9) | 32 (30.2) | 36 (32.7) | 36 (32.7) |
| Apixaban, n (%) | 114 (35.0) | 39 (36.8) | 38 (34.5) | 37 (33.6) |
| NOAC dosing | ||||
| Underdosinga, n (%) | 56 (17.2) | 21 (19.8) | 16 (14.5) | 19 (17.3) |
| Labelled use, n (%) | 269 (82.5) | 85 (80.2) | 93 (84.5) | 91 (82.7) |
| Overdosingb, n (%) | 1 (0.3) | 0 | 1 (0.9) | 0 |
| Comorbidities | ||||
| Heart failure, n (%) | 43 (13.2) | 11 (10.4) | 16 (14.5) | 16 (14.5) |
| Hypertension, n (%) | 140 (42.9) | 45 (42.5) | 45 (40.9) | 50 (45.5) |
| Diabetes, n (%) | 47 (14.4) | 10 (9.4) | 16 (14.5) | 21 (19.1) |
| Stroke/TIA, n (%) | 40 (12.3) | 12 (11.3) | 17 (15.5) | 11 (10.0) |
| Vascular disease, n (%) | 25 (7.7) | 8 (7.6) | 11 (10.0) | 6 (5.5) |
| CHA2DS2-VASc score | 1.7 ± 1.5 | 1.6 ± 1.4 | 1.7 ± 1.5 | 1.7 ± 1.4 |
| Echocardiographic measures | ||||
| LA dimension (mm) | 41.2 ± 6.7 | 41.0 ± 7.6 | 41.0 ± 6.0 | 41.6 ± 6.6 |
| LA volume index (mL/m2) | 39.1 ± 14.7 | 39.4 ± 13.2 | 39.2 ± 16.3 | 38.8 ± 14.6 |
| LVEDD (mm) | 49.8 ± 4.6 | 49.8 ± 4.5 | 49.3 ± 4.5 | 50.3 ± 4.8 |
| LV ejection fraction (%) | 61.3 ± 9.5 | 61.2 ± 9.0 | 61.3 ± 10.3 | 61.3 ± 9.5 |
| E/Em | 9.8 ± 4.0 | 9.7 ± 4.3 | 10.1 ± 4.2 | 9.5 ± 3.5 |
| . | Total (n = 326) . | UI (n = 106) . | SDS (n = 110) . | 24S (n = 110) . |
|---|---|---|---|---|
| Age (years) | 58.3 ± 11.3 | 58.6 ± 11.7 | 57.9 ± 11.1 | 58.4 ± 11.3 |
| Male, n (%) | 243 (74.5) | 81 (76.4) | 79 (71.8) | 83 (75.5) |
| Paroxysmal AF, n (%) | 202 (62.0) | 67 (63.2) | 74 (67.3) | 61 (55.5) |
| AF duration (months) | 47.2 ± 51.7 | 47.3 ± 49.8 | 53.1 ± 58.3 | 41.3 ± 45.9 |
| BMI (kg/m2) | 25.3 ± 3.4 | 26.1 ± 3.7 | 24.7 ± 3.1 | 25.3 ± 3.3 |
| Body weight (kg) | 73.6 ± 18.4 | 73.9 ± 15.5 | 71.3 ± 17.8 | 75.6 ± 21.2 |
| CrCl (mL/min) | 93.2 ± 41.8 | 97.5 ± 54.1 | 90.9 ± 31.5 | 91.4 ± 37.0 |
| NOAC type | ||||
| Dabigatran, n (%) | 108 (33.1) | 35 (33.0) | 36 (32.7) | 37 (33.6) |
| Rivaroxaban, n (%) | 104 (31.9) | 32 (30.2) | 36 (32.7) | 36 (32.7) |
| Apixaban, n (%) | 114 (35.0) | 39 (36.8) | 38 (34.5) | 37 (33.6) |
| NOAC dosing | ||||
| Underdosinga, n (%) | 56 (17.2) | 21 (19.8) | 16 (14.5) | 19 (17.3) |
| Labelled use, n (%) | 269 (82.5) | 85 (80.2) | 93 (84.5) | 91 (82.7) |
| Overdosingb, n (%) | 1 (0.3) | 0 | 1 (0.9) | 0 |
| Comorbidities | ||||
| Heart failure, n (%) | 43 (13.2) | 11 (10.4) | 16 (14.5) | 16 (14.5) |
| Hypertension, n (%) | 140 (42.9) | 45 (42.5) | 45 (40.9) | 50 (45.5) |
| Diabetes, n (%) | 47 (14.4) | 10 (9.4) | 16 (14.5) | 21 (19.1) |
| Stroke/TIA, n (%) | 40 (12.3) | 12 (11.3) | 17 (15.5) | 11 (10.0) |
| Vascular disease, n (%) | 25 (7.7) | 8 (7.6) | 11 (10.0) | 6 (5.5) |
| CHA2DS2-VASc score | 1.7 ± 1.5 | 1.6 ± 1.4 | 1.7 ± 1.5 | 1.7 ± 1.4 |
| Echocardiographic measures | ||||
| LA dimension (mm) | 41.2 ± 6.7 | 41.0 ± 7.6 | 41.0 ± 6.0 | 41.6 ± 6.6 |
| LA volume index (mL/m2) | 39.1 ± 14.7 | 39.4 ± 13.2 | 39.2 ± 16.3 | 38.8 ± 14.6 |
| LVEDD (mm) | 49.8 ± 4.6 | 49.8 ± 4.5 | 49.3 ± 4.5 | 50.3 ± 4.8 |
| LV ejection fraction (%) | 61.3 ± 9.5 | 61.2 ± 9.0 | 61.3 ± 10.3 | 61.3 ± 9.5 |
| E/Em | 9.8 ± 4.0 | 9.7 ± 4.3 | 10.1 ± 4.2 | 9.5 ± 3.5 |
All variables were not significantly different between the three treatment groups (P > 0.05).
24S, 24-hour skipped; AF, atrial fibrillation; BMI, body mass index; CHA2DS2-VASc, congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, prior stroke, transient ischaemic attack, or thromboembolism, vascular disease, age 65–74 years, sex category (female); CrCl, creatinine clearance; E/Em, early mitral inflow velocity over the early diastolic mitral annular velocity; LA, left atrium; LV, left ventricle; LVEDD, left ventricular end-diastolic dimension; NOAC, non-vitamin K antagonist oral anticoagulants; SDS, single-dose skip; TIA, transient ischaemic attack; UI, uninterrupted.
Main reasons for underdosing were concomitant use of antiplatelets and the eGFR change.
The reasons for overdosing were a deranged renal function and reduced body weight.
The relevant procedure-related variables are outlined in Table 2. The total procedure time, fluoroscopic time, and ablation time were comparable among the three groups (P > 0.05 for all). All patients received heparin during the catheter ablation procedure. The mean ACT level did not differ significantly among the treatment groups (P = 0.139). Interestingly, the mean total number of units of heparin administered to achieve the target ACT range was highest in the 24S group and lowest in the UI group (22093 ± 6974 IU and 18740 ± 5726 IU, respectively; P < 0.001).
Procedure outcomes
| . | Total (n = 326) . | UI (n = 106) . | SDS (n = 110) . | 24S (n = 110) . | P-value . |
|---|---|---|---|---|---|
| Total procedure time (min) | 190.9 ± 59.5 | 184.8 ± 56.4 | 190.2 ± 60.8 | 197.6 ± 60.9 | 0.318 |
| Fluoroscopic time (min) | 35.0 ± 15.4 | 33.0 ± 13.8 | 35.4 ± 12.6 | 36.7 ± 18.9 | 0.205 |
| Ablation time (s) | 4548.1 ± 1838.3 | 4421.5 ± 1751.1 | 4469.6 ± 2025.3 | 4748.7 ± 1720.0 | 0.367 |
| Heparin dose (units) | 20343.3 ± 6191.2 | 18740.4 ± 5726.4 | 20135.5 ± 5324.6 | 22092.6 ± 6974.0 | <0.001 |
| Activated clotting time (s) | 347.5 ± 30.7 | 351.7 ± 36.5 | 347.5 ± 29.8 | 343.3 ± 24.6 | 0.139 |
| Δ Haemoglobin (g/dL) | −1.77 ± 1.67 | −1.50 ± 1.64 | −1.84 ± 1.07 | −1.97 ± 2.13 | 0.114 |
| Complications, n (%) | 13 (4.0) | 2 (1.9) | 5 (4.5) | 6 (5.5) | 0.381 |
| Cardiac tamponade, n (%) | 3 (0.9) | 1 (0.9) | 0 | 2 (1.8) | |
| Pericardial effusion, n (%) | 2 (0.6) | 1 (0.9) | 1 (0.9) | 0 | |
| Arteriovenous fistula, n (%) | 2 (0.6) | 0 | 2 (1.8) | 0 | |
| Phrenic nerve damage, n (%) | 1 (0.3) | 0 | 0 | 1 (0.9) | |
| Sinus dysfunction, n (%) | 4 (1.2) | 0 | 1 (0.9) | 3 (2.7) | |
| Complete AV block, n (%) | 1 (0.3) | 0 | 1 (0.9) | 0 | |
| Stroke, n (%) | 0 | 0 | 0 | 0 |
| . | Total (n = 326) . | UI (n = 106) . | SDS (n = 110) . | 24S (n = 110) . | P-value . |
|---|---|---|---|---|---|
| Total procedure time (min) | 190.9 ± 59.5 | 184.8 ± 56.4 | 190.2 ± 60.8 | 197.6 ± 60.9 | 0.318 |
| Fluoroscopic time (min) | 35.0 ± 15.4 | 33.0 ± 13.8 | 35.4 ± 12.6 | 36.7 ± 18.9 | 0.205 |
| Ablation time (s) | 4548.1 ± 1838.3 | 4421.5 ± 1751.1 | 4469.6 ± 2025.3 | 4748.7 ± 1720.0 | 0.367 |
| Heparin dose (units) | 20343.3 ± 6191.2 | 18740.4 ± 5726.4 | 20135.5 ± 5324.6 | 22092.6 ± 6974.0 | <0.001 |
| Activated clotting time (s) | 347.5 ± 30.7 | 351.7 ± 36.5 | 347.5 ± 29.8 | 343.3 ± 24.6 | 0.139 |
| Δ Haemoglobin (g/dL) | −1.77 ± 1.67 | −1.50 ± 1.64 | −1.84 ± 1.07 | −1.97 ± 2.13 | 0.114 |
| Complications, n (%) | 13 (4.0) | 2 (1.9) | 5 (4.5) | 6 (5.5) | 0.381 |
| Cardiac tamponade, n (%) | 3 (0.9) | 1 (0.9) | 0 | 2 (1.8) | |
| Pericardial effusion, n (%) | 2 (0.6) | 1 (0.9) | 1 (0.9) | 0 | |
| Arteriovenous fistula, n (%) | 2 (0.6) | 0 | 2 (1.8) | 0 | |
| Phrenic nerve damage, n (%) | 1 (0.3) | 0 | 0 | 1 (0.9) | |
| Sinus dysfunction, n (%) | 4 (1.2) | 0 | 1 (0.9) | 3 (2.7) | |
| Complete AV block, n (%) | 1 (0.3) | 0 | 1 (0.9) | 0 | |
| Stroke, n (%) | 0 | 0 | 0 | 0 |
24S, 24-hour skipped; AV, atrioventricular; SDS, single-dose skip; UI, uninterrupted.
Procedure outcomes
| . | Total (n = 326) . | UI (n = 106) . | SDS (n = 110) . | 24S (n = 110) . | P-value . |
|---|---|---|---|---|---|
| Total procedure time (min) | 190.9 ± 59.5 | 184.8 ± 56.4 | 190.2 ± 60.8 | 197.6 ± 60.9 | 0.318 |
| Fluoroscopic time (min) | 35.0 ± 15.4 | 33.0 ± 13.8 | 35.4 ± 12.6 | 36.7 ± 18.9 | 0.205 |
| Ablation time (s) | 4548.1 ± 1838.3 | 4421.5 ± 1751.1 | 4469.6 ± 2025.3 | 4748.7 ± 1720.0 | 0.367 |
| Heparin dose (units) | 20343.3 ± 6191.2 | 18740.4 ± 5726.4 | 20135.5 ± 5324.6 | 22092.6 ± 6974.0 | <0.001 |
| Activated clotting time (s) | 347.5 ± 30.7 | 351.7 ± 36.5 | 347.5 ± 29.8 | 343.3 ± 24.6 | 0.139 |
| Δ Haemoglobin (g/dL) | −1.77 ± 1.67 | −1.50 ± 1.64 | −1.84 ± 1.07 | −1.97 ± 2.13 | 0.114 |
| Complications, n (%) | 13 (4.0) | 2 (1.9) | 5 (4.5) | 6 (5.5) | 0.381 |
| Cardiac tamponade, n (%) | 3 (0.9) | 1 (0.9) | 0 | 2 (1.8) | |
| Pericardial effusion, n (%) | 2 (0.6) | 1 (0.9) | 1 (0.9) | 0 | |
| Arteriovenous fistula, n (%) | 2 (0.6) | 0 | 2 (1.8) | 0 | |
| Phrenic nerve damage, n (%) | 1 (0.3) | 0 | 0 | 1 (0.9) | |
| Sinus dysfunction, n (%) | 4 (1.2) | 0 | 1 (0.9) | 3 (2.7) | |
| Complete AV block, n (%) | 1 (0.3) | 0 | 1 (0.9) | 0 | |
| Stroke, n (%) | 0 | 0 | 0 | 0 |
| . | Total (n = 326) . | UI (n = 106) . | SDS (n = 110) . | 24S (n = 110) . | P-value . |
|---|---|---|---|---|---|
| Total procedure time (min) | 190.9 ± 59.5 | 184.8 ± 56.4 | 190.2 ± 60.8 | 197.6 ± 60.9 | 0.318 |
| Fluoroscopic time (min) | 35.0 ± 15.4 | 33.0 ± 13.8 | 35.4 ± 12.6 | 36.7 ± 18.9 | 0.205 |
| Ablation time (s) | 4548.1 ± 1838.3 | 4421.5 ± 1751.1 | 4469.6 ± 2025.3 | 4748.7 ± 1720.0 | 0.367 |
| Heparin dose (units) | 20343.3 ± 6191.2 | 18740.4 ± 5726.4 | 20135.5 ± 5324.6 | 22092.6 ± 6974.0 | <0.001 |
| Activated clotting time (s) | 347.5 ± 30.7 | 351.7 ± 36.5 | 347.5 ± 29.8 | 343.3 ± 24.6 | 0.139 |
| Δ Haemoglobin (g/dL) | −1.77 ± 1.67 | −1.50 ± 1.64 | −1.84 ± 1.07 | −1.97 ± 2.13 | 0.114 |
| Complications, n (%) | 13 (4.0) | 2 (1.9) | 5 (4.5) | 6 (5.5) | 0.381 |
| Cardiac tamponade, n (%) | 3 (0.9) | 1 (0.9) | 0 | 2 (1.8) | |
| Pericardial effusion, n (%) | 2 (0.6) | 1 (0.9) | 1 (0.9) | 0 | |
| Arteriovenous fistula, n (%) | 2 (0.6) | 0 | 2 (1.8) | 0 | |
| Phrenic nerve damage, n (%) | 1 (0.3) | 0 | 0 | 1 (0.9) | |
| Sinus dysfunction, n (%) | 4 (1.2) | 0 | 1 (0.9) | 3 (2.7) | |
| Complete AV block, n (%) | 1 (0.3) | 0 | 1 (0.9) | 0 | |
| Stroke, n (%) | 0 | 0 | 0 | 0 |
24S, 24-hour skipped; AV, atrioventricular; SDS, single-dose skip; UI, uninterrupted.
Efficacy and safety outcomes
The incidence of major bleeding during and up to 1 month after the ablation and post-procedural reduction in the haemoglobin level did not significantly differ among the treatment groups (P > 0.05 for all). The number of major bleeding events in the three groups was as follows: cardiac tamponade requiring pericardial drainage, 1 event in the UI group and 2 events in the 24S group; and pericardial effusion, 1 event in the UI group and 1 event in the SDS group. There were no stroke events, systemic embolisms, or transient ischaemic attacks in all the three groups.
The numbers of adverse events were similarly low in the three treatment groups. Two femoral arteriovenous fistula events occurred in the SDS group, and 1 phrenic nerve damage event in the 24S group. One sinus dysfunction event occurred in the SDS group and 3 events in the 24S group. One occurrence of complete atrioventricular block was reported in the SDS group. No fatal events were reported in any of the groups.
Differences between the non-vitamin K antagonist oral anticoagulants
We additionally compared the efficacy and safety outcomes according to the type of NOACs. Although they were not randomized, there was no significant difference in the baseline characteristics among the different NOAC groups (Supplementary material online, Table S1). Supplementary material online, Table S2 shows the procedural outcomes according to the type of NOAC. The mean total units of heparin administered did not differ significantly between the different NOAC groups (P = 0.441). However, the mean ACT level was highest in rivaroxaban users and lowest in apixaban users (P = 0.026). The incidence of major bleeding and a post-procedural reduction in the haemoglobin level were comparable among the different NOACs (P > 0.05 for all). The number of other adverse events was similarly low and also did not significantly differ among the three NOAC groups.
Discussion
Main findings
This multicentre, prospective RCT assessed the safety and efficacy of UI, procedure day SDS, and 24 h skipped NOACs in patients undergoing AF ablation. The incidence of major bleeding during and up to 1 month after the ablation and the post-procedural reduction in the haemoglobin level did not significantly differ among the treatment groups and different NOACs. The intra-procedural heparin requirement was higher in the 24S group, and the mean ACT level was comparable among the groups. There were no thrombo-embolic complications or fatal events in any of the three groups.
Usefulness of non-vitamin K antagonist oral anticoagulants in the periprocedural period of atrial fibrillation catheter ablation
The use of NOACs is steadily increasing among AF patients undergoing RFCA.14 Several small observational studies and meta- analyses15–17 have suggested a similar safety and efficacy profile for rivaroxaban, apixaban, and the direct thrombin inhibitor, dabigatran, compared to warfarin. In a subanalysis of the ROCKET AF study,18 a relatively small group of patients undergoing RFCA or cardioversion had similar outcomes when receiving either rivaroxaban or warfarin. Interpretation of these data has been limited by small samples, small numbers of events, and non-randomized or retrospective study designs. The VENTURE AF trial,9 the first prospective randomized trial of an NOAC in AF RFCA, showed that patients undergoing catheter ablation of non-valvular AF had similar safety outcomes with UI rivaroxaban and UI warfarin therapy. The limitation of this trial was the small sample size and low number of major bleeding and thrombo-embolic events. Subsequently, the RE-CIRCUIT trial10 reported that UI dabigatran was associated with significantly fewer bleeding complications than UI warfarin in patients undergoing ablation of AF. The possible mechanism of reducing bleeding complications with dabigatran, suggested by the authors, may be related to the more specific mechanism of action and shorter half-life of dabigatran as compared with warfarin. Recently, the AEIOU trial19 also showed that both UI and minimally interrupted apixaban were associated with a low rate of thrombo-embolic events, and the rates of both major and clinically significant bleeding were similar to that of UI warfarin. In the present trial, using NOACs at the time of the AF ablation was associated with a very low rate of bleeding and/or thrombo-embolic events, and the rate of major bleeding events and a post-procedural haemoglobin reduction did not significantly differ among the treatment groups and different NOACs. This finding reinforces the current expert consensus recommendations on the use of NOACs at the time of AF ablation.4
In patients undergoing AF ablation, uninterrupted, single dose skipped, and 24-hour skipped NOACs exhibited a comparable safety, and the intra-procedural heparin requirement was higher in the 24S group.
Clinical implications
The major concern of UI NOACs during AF catheter ablation might be the risk of bleeding, especially life-threatening bleeding such as cardiac tamponade. The <2% rate of major bleeding seen in this study was also similar to that reported from a recent meta-analysis of 19 studies with 7996 patients.20 In that report, periprocedural NOAC therapy was as effective as continuous warfarin therapy for preventing thromboembolisms and had a lower incidence of bleeding complications. In addition, interrupted NOAC therapy during the periprocedural period might result in a lower incidence of bleeding complications compared to continuous NOAC therapy. In our study, a time of the NOAC discontinuation within 24 h of the ablation procedure did not affect the patients’ thrombo-embolic or bleeding risk. But, for the patients with persistent AF with a CHA2DS2-VASc score of ≥2 in the 24S group, we used low molecular weight heparin bridging on the evening the day before the procedure. A systematic review and meta-analysis suggested that periprocedural heparin bridging appears to be at an increased risk for bleeding complications21; however, in the present study, both cardiac tamponade events occurred in the subgroup in the 24S group that did not use low molecular weight heparin. In addition, there were no statistically significant differences in the periprocedural heparin dosing and ACTs depending on the use of low molecular weight heparin among the 24S group.
In the RE-CIRCUIT trial, one advantage of the periprocedural use of dabigatran was the availability of a reversal agent that could achieve an immediate reversal of the anticoagulant effect.3 However, up until now, no reversal agent has become commercially available for factor Xa inhibitors. Furthermore, in Asians, more attention is required during periprocedural anticoagulation therapy because of their vulnerability to an anticoagulant-related bleeding risk.11 There were three cases with cardiac tamponade requiring pericardial catheter drainage, and another two cases with pericardial effusions that resolved only with conservative treatment without drainage. No NOAC reversal agents were used in our cases, however, the availability of a reversal agent that could achieve an immediate reversal of the anticoagulant effect could be a potential advantage of the periprocedural NOAC choice. With the assurance that the risk of a stroke or thrombo-embolic events is not increased, interruption of one dose of NOACs during the periprocedural period can be a good option to reduce any potential bleeding risk of UI-NOACs and to avoid the inconvenience of low molecular weight heparin injections in the 24S group.
Limitations
There were several limitations to the present study. First, the sample size was not large enough to present the differences in the major outcomes. The current exploratory sample size reflects the infeasibility of conducting a fully powered study based on standard calculations. Although the low incidence of events likely represented a population of highly experienced electrophysiologists performing the RFCA, this element could have reduced the sensitivity of the results. Second, the open-label design could have introduced a bias related to the knowledge of the treatment allocation. Moreover, since the half-life of each NOAC differs, the effects of the drug interruption may differ even if the patients belong to the same treatment group. In addition, the comparisons between the different NOAC groups were not randomized, but assigned in order after randomization. Although we observed no strokes or transient ischaemic attacks, a clinical stroke would not reflect a subclinical brain injury during ablation. However, no routine MRI was performed for the purpose of detecting asymptomatic cranial lesions following the ablation in our study. All the patients underwent preablation TOE to rule out any LA thrombi, and the results of this study cannot be directly applied to procedures without first excluding any LA thrombi by imaging and providing adequate anticoagulation. Despite these limitations, our study, to the best of our knowledge, was the first trial to assess the safety and efficacy of the different dosing regimens of NOACs in patients undergoing AF ablation.
Conclusion
In patients undergoing AF ablation, uninterrupted NOACs and skipping single or double doses of NOACs exhibited a comparable efficacy and safety, regardless of the type of NOAC. Given these findings, all three NOAC strategies are clinically relevant for AF ablation.
Acknowledgements
We thank Mr John Martin for his linguistic assistance.
Funding
Korea Health 21R&D Project, Ministry of Health and Welfare (HI18C0070); Basic Science Research Program run by the National Research Foundation of Korea (NRF) (NRF-2017R1A2B4003983; 2017R1C1B1008292) which is funded by the Ministry of Science, ICT & Future Planning (MSIP).
Conflict of interest: none declared.
References