-
PDF
- Split View
-
Views
-
Cite
Cite
Lucia Mazzolai, Victor Aboyans, Walter Ageno, Giancarlo Agnelli, Adriano Alatri, Rupert Bauersachs, Marjolein P A Brekelmans, Harry R Büller, Antoine Elias, Dominique Farge, Stavros Konstantinides, Gualtiero Palareti, Paolo Prandoni, Marc Righini, Adam Torbicki, Charalambos Vlachopoulos, Marianne Brodmann, Diagnosis and management of acute deep vein thrombosis: a joint consensus document from the European Society of Cardiology working groups of aorta and peripheral vascular diseases and pulmonary circulation and right ventricular function, European Heart Journal, Volume 39, Issue 47, 14 December 2018, Pages 4208–4218, https://doi.org/10.1093/eurheartj/ehx003
Close - Share Icon Share
Introduction
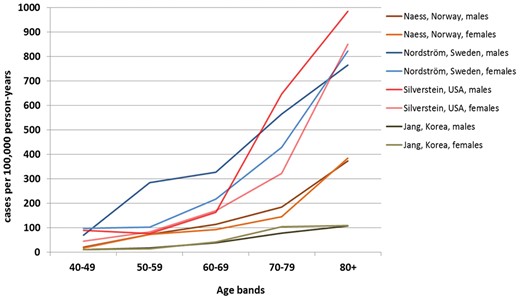
Venous thromboembolism (VTE) incidence increases sharply with age (Figure1) and appears steady over the last 25 years, despite preventive strategies.1 Women are more often affected at younger ages; this ratio reverses in the elderly.2 Incidence is similar in Blacks but lower in Asians.3 Almost two-thirds of VTE cases are isolated deep vein thromboses (DVTs), and 80% are proximal.4
Recent European population studies reported DVT incidence of 70–140 cases/100,000 person-year.5
Deep vein thrombosis are mostly secondary to predisposing factors common with pulmonary embolism (PE) (webtable 1).6 Distal (below knee) DVTs are more frequently related to transient situations while proximal ones to chronic conditions.7 In 25–50% of first DVT episodes, no predisposing factor is identified.
In patients with DVT without PE, short-term mortality rates of 2–5% were reported, more frequent in proximal than distal DVT.7 Recurrence risk is high, especially within first 6 months.8
Early- and mid-term complications include thrombosis extension, and PE and DVT recurrence (see Supplementary material online, only section).
Long-term complications include post-thrombotic syndrome (PTS), defined as chronic venous symptoms and/or signs secondary to DVT. It represents the most frequent chronic DVT complication, occurring in 30–50% of patients within 2 years after proximal DVT.9 In 5–10% of cases, PTS is severe.9 Previous ipsilateral DVT, proximal location (ilio-femoral > popliteal), and residual veins obstruction are most significant PTS risk factors. Obesity and poor INR control during the first 3-months treatment are additional independent risk factors.10
Villalta score is used for PTS diagnosis and treatment evaluation (Table1).11
Villalta score11
| Symptoms and Clinical signs . | None . | Mild . | Moderate . | Severe . |
|---|---|---|---|---|
| Symptoms | ||||
| Pain | 0 points | 1 points | 2 points | 3 points |
| Cramps | 0 points | 1 points | 2 points | 3 points |
| Haeviness | 0 points | 1 points | 2 points | 3 points |
| Paresthesia | 0 points | 1 points | 2 points | 3 points |
| Pruritus | 0 points | 1 points | 2 points | 3 points |
| Clinical signs | ||||
| Pretibial edema | 0 points | 1 points | 2 points | 3 points |
| Skin induration | 0 points | 1 points | 2 points | 3 points |
| Hyperpigmentation | 0 points | 1 points | 2 points | 3 points |
| Redness | 0 points | 1 points | 2 points | 3 points |
| Venous ectasia | 0 points | 1 points | 2 points | 3 points |
| Pain on calf compression | 0 points | 1 points | 2 points | 3 points |
| Venous ulcer | Absent | Present |
| Symptoms and Clinical signs . | None . | Mild . | Moderate . | Severe . |
|---|---|---|---|---|
| Symptoms | ||||
| Pain | 0 points | 1 points | 2 points | 3 points |
| Cramps | 0 points | 1 points | 2 points | 3 points |
| Haeviness | 0 points | 1 points | 2 points | 3 points |
| Paresthesia | 0 points | 1 points | 2 points | 3 points |
| Pruritus | 0 points | 1 points | 2 points | 3 points |
| Clinical signs | ||||
| Pretibial edema | 0 points | 1 points | 2 points | 3 points |
| Skin induration | 0 points | 1 points | 2 points | 3 points |
| Hyperpigmentation | 0 points | 1 points | 2 points | 3 points |
| Redness | 0 points | 1 points | 2 points | 3 points |
| Venous ectasia | 0 points | 1 points | 2 points | 3 points |
| Pain on calf compression | 0 points | 1 points | 2 points | 3 points |
| Venous ulcer | Absent | Present |
Points are summed into a total score (range 0–33). Post Thrombotic syndrome (PTS) is defined by a total score of ≥5 or the presence of a venous ulcer. PTS is classified as mild if Villalta score is 5–9, moderate if 10–14, and severe if ≥15 or venous ulcer is present.
Villalta score11
| Symptoms and Clinical signs . | None . | Mild . | Moderate . | Severe . |
|---|---|---|---|---|
| Symptoms | ||||
| Pain | 0 points | 1 points | 2 points | 3 points |
| Cramps | 0 points | 1 points | 2 points | 3 points |
| Haeviness | 0 points | 1 points | 2 points | 3 points |
| Paresthesia | 0 points | 1 points | 2 points | 3 points |
| Pruritus | 0 points | 1 points | 2 points | 3 points |
| Clinical signs | ||||
| Pretibial edema | 0 points | 1 points | 2 points | 3 points |
| Skin induration | 0 points | 1 points | 2 points | 3 points |
| Hyperpigmentation | 0 points | 1 points | 2 points | 3 points |
| Redness | 0 points | 1 points | 2 points | 3 points |
| Venous ectasia | 0 points | 1 points | 2 points | 3 points |
| Pain on calf compression | 0 points | 1 points | 2 points | 3 points |
| Venous ulcer | Absent | Present |
| Symptoms and Clinical signs . | None . | Mild . | Moderate . | Severe . |
|---|---|---|---|---|
| Symptoms | ||||
| Pain | 0 points | 1 points | 2 points | 3 points |
| Cramps | 0 points | 1 points | 2 points | 3 points |
| Haeviness | 0 points | 1 points | 2 points | 3 points |
| Paresthesia | 0 points | 1 points | 2 points | 3 points |
| Pruritus | 0 points | 1 points | 2 points | 3 points |
| Clinical signs | ||||
| Pretibial edema | 0 points | 1 points | 2 points | 3 points |
| Skin induration | 0 points | 1 points | 2 points | 3 points |
| Hyperpigmentation | 0 points | 1 points | 2 points | 3 points |
| Redness | 0 points | 1 points | 2 points | 3 points |
| Venous ectasia | 0 points | 1 points | 2 points | 3 points |
| Pain on calf compression | 0 points | 1 points | 2 points | 3 points |
| Venous ulcer | Absent | Present |
Points are summed into a total score (range 0–33). Post Thrombotic syndrome (PTS) is defined by a total score of ≥5 or the presence of a venous ulcer. PTS is classified as mild if Villalta score is 5–9, moderate if 10–14, and severe if ≥15 or venous ulcer is present.
Diagnosis
Deep vein thrombosis without pulmonary embolism symptoms
Clinical signs and symptoms are highly variable and unspecific but remain the cornerstone of diagnostic strategy. Symptoms include pain, swelling, increased skin veins visibility, erythema, and cyanosis accompanied by unexplained fever.
Probability assessment and d-dimer testing
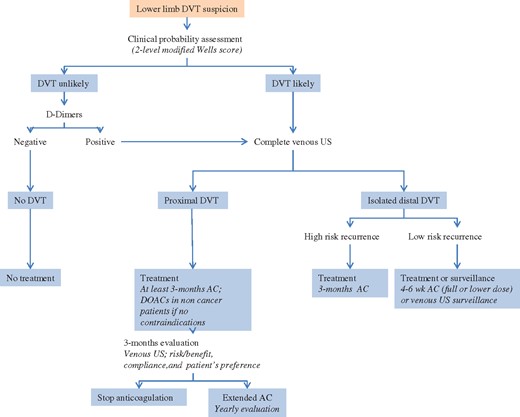
Pre-test probability assessment is the first step in the diagnostic algorithm of DVT suspicion (Figure2). Sensitivity and specificity of clinical symptoms are low when considered individually; however, their combination, using prediction rules, allows pre-test clinical probability classification into two- (DVT unlikely or likely) or three-categories (low-, intermediate-, or high-clinical probability) corresponding to increasing disease prevalence.12,13 Wells score has been widely validated and can be applied both to out- and inpatients (Table2). The experts’ panel favours the modified two-level pre-test probability as it is more straightforward.14
| Clinical variable . | Points . |
|---|---|
| Active cancer (treatment ongoing or within previous 6 months or palliative) | +1 |
| Paralysis, paresis or recent plaster immobilization of the lower extremities | +1 |
| Recently bedridden for 3 days or more, or major surgery within the previous 12 weeks requiring general or regional anesthesia | +1 |
| Localized tenderness along the distribution of the deep venous system | +1 |
| Entire leg swelling | +1 |
| Calf swelling at least 3 cm larger than that on the asymptomatic leg (measured 10 cm below the tibial tuberosity) | +1 |
| Pitting edema confined to the symptomatic leg | +1 |
| Collateral superficial veins (non varicose) | +1 |
| Previously documented DVT | +1 |
| Alternative diagnosis at least as likely as DVT | −2 |
| Three-level Wells score | |
| Low | <1 |
| Intermediate | 1–2 |
| High | >2 |
| Two-level Wells score | |
| Unlikely | ≤1 |
| Likely | ≥2 |
| Clinical variable . | Points . |
|---|---|
| Active cancer (treatment ongoing or within previous 6 months or palliative) | +1 |
| Paralysis, paresis or recent plaster immobilization of the lower extremities | +1 |
| Recently bedridden for 3 days or more, or major surgery within the previous 12 weeks requiring general or regional anesthesia | +1 |
| Localized tenderness along the distribution of the deep venous system | +1 |
| Entire leg swelling | +1 |
| Calf swelling at least 3 cm larger than that on the asymptomatic leg (measured 10 cm below the tibial tuberosity) | +1 |
| Pitting edema confined to the symptomatic leg | +1 |
| Collateral superficial veins (non varicose) | +1 |
| Previously documented DVT | +1 |
| Alternative diagnosis at least as likely as DVT | −2 |
| Three-level Wells score | |
| Low | <1 |
| Intermediate | 1–2 |
| High | >2 |
| Two-level Wells score | |
| Unlikely | ≤1 |
| Likely | ≥2 |
| Clinical variable . | Points . |
|---|---|
| Active cancer (treatment ongoing or within previous 6 months or palliative) | +1 |
| Paralysis, paresis or recent plaster immobilization of the lower extremities | +1 |
| Recently bedridden for 3 days or more, or major surgery within the previous 12 weeks requiring general or regional anesthesia | +1 |
| Localized tenderness along the distribution of the deep venous system | +1 |
| Entire leg swelling | +1 |
| Calf swelling at least 3 cm larger than that on the asymptomatic leg (measured 10 cm below the tibial tuberosity) | +1 |
| Pitting edema confined to the symptomatic leg | +1 |
| Collateral superficial veins (non varicose) | +1 |
| Previously documented DVT | +1 |
| Alternative diagnosis at least as likely as DVT | −2 |
| Three-level Wells score | |
| Low | <1 |
| Intermediate | 1–2 |
| High | >2 |
| Two-level Wells score | |
| Unlikely | ≤1 |
| Likely | ≥2 |
| Clinical variable . | Points . |
|---|---|
| Active cancer (treatment ongoing or within previous 6 months or palliative) | +1 |
| Paralysis, paresis or recent plaster immobilization of the lower extremities | +1 |
| Recently bedridden for 3 days or more, or major surgery within the previous 12 weeks requiring general or regional anesthesia | +1 |
| Localized tenderness along the distribution of the deep venous system | +1 |
| Entire leg swelling | +1 |
| Calf swelling at least 3 cm larger than that on the asymptomatic leg (measured 10 cm below the tibial tuberosity) | +1 |
| Pitting edema confined to the symptomatic leg | +1 |
| Collateral superficial veins (non varicose) | +1 |
| Previously documented DVT | +1 |
| Alternative diagnosis at least as likely as DVT | −2 |
| Three-level Wells score | |
| Low | <1 |
| Intermediate | 1–2 |
| High | >2 |
| Two-level Wells score | |
| Unlikely | ≤1 |
| Likely | ≥2 |
Proposed deep vein thrombosis diagnostic and management algorithm. AC, anticoagulation; DOAC, direct oral anticoagulant.
Normal d-dimers render DVT unlikely,15 however, d-dimers have low specificity. Quantitative ELISA or ELISA-derived assays (>95% sensitivity) allow ruling out DVT in patients with DVT ‘unlikely’. Negative ELISA d-dimer can exclude DVT without further testing in 30% of patients,16 with 3-month thromboembolic risk <1% without treatment.13 Quantitative latex-derived and whole-blood agglutination assay have lower sensitivity (85–90%).17 In patients with ‘likely’ DVT, d-Dimer testing is not necessary: imaging is required. Therapeutic anticoagulation should be initiated, if not contraindicated, in patients with DVT ‘likely’ until imaging.
Imaging
Venous ultrasound (VUS) is the first line DVT imaging modality (other imaging: see Supplementary material online, only section). It is based on B-mode, combined or not with color-Doppler US, and power imaging techniques. DVT diagnostic criteria are cross-sectional vein incompressibility, direct thrombus imaging with vein enlargement, and abnormal spectral and color-Doppler flow. VUS can be performed by examining popliteal and common femoral veins only [2-point/2-region compression venous ultrasonography (CUS) or limited CUS], or by extended imaging of inferior vena cava, iliac and femoral veins, and calf veins (whole-leg VUS or complete VUS). There are controversies as to whether explore symptomatic leg only, or both.18,19
In clinically suspected DVT, VUS provides overall sensitivity of 94.2% for proximal, and 63.5% for isolated distal DVT, with an overall specificity of 93.8%.20 Combination with color-Doppler US increases sensitivity but lowers specificity.20 When DVT is suspected (without PE symptoms), anticoagulation may be safely withheld in patients with a single normal complete VUS. Same is true for limited CUS provided it can be repeated, and integrated within a diagnostic strategy including clinical probability, and d-dimer assessment.21 Overall 3-month VTE incidence rate after negative complete VUS is 0.57%,22 but both methods are reported to be equivalent in randomized trials.23,24 Complete VUS may be helpful to explain patient’s complaint by providing up to 42% alternative diagnosis.25 Point-of care US performed by emergency physicians using limited CUS has shown good performance (96.1% sensitivity, 96.8% specificity)26 and may be useful if vascular laboratories are not available 24/7, provided its integration in a validated diagnostic strategy.27
In patients with clinically suspected recurrent DVT: comparison of test results with baseline imaging at discontinuation of anticoagulation can safely rule out diagnosis of recurrence.28 A 2- or 4-mm29–31 increase in vein diameter between two measurements at the common femoral and popliteal veins, after full compression, is the most validated US criterion.
Deep vein thrombosis with pulmonary embolism symptoms
Diagnostic approach is described in corresponding 2014 European Society of Cardiology (ESC) Guidelines.6 Proximal DVT confirmation in a normotensive patient with suspected PE essentially confirms VTE and justifies anticoagulation as after formal PE diagnosis. In unstable patients with right ventricular overload but no possibility to confirm PE, CUS showing proximal DVT facilitates initiation of reperfusion therapy. CUS diagnostic yield is high in the presence of clinical DVT signs.32 Among unselected PE patients, proximal DVT at CUS is found in 1/7 patients.33 Proximal DVT has high specificity and may justify treatment even if pulmonary CT is negative.6 While negative CUS cannot exclude PE, it can justify withholding anticoagulation in patients with non-diagnostic ventilation/perfusion scan and PE-unlikely.16,34,35
In symptomatic patients with isolated sub-segmental PE or incidental asymptomatic PE, concomitant DVT justifies anticoagulation.36,37 Deep vein thrombosis imaging may also be useful if secondarily a patient is suspected of VTE recurrence with DVT signs. Moreover, presence of concomitant DVT has been suggested as an independent 30-days death risk factor following PE.38
Consensus statement: diagnosis
Clinical prediction rule (two-level modified Wells score) is recommended to stratify patients with suspected lower limb DVT.
ELISA d-dimer measurement is recommended in ‘unlikely’ clinical probability patients to exclude DVT.
Venous US is recommended as first line imaging method for DVT diagnosis.
Venous CT scan should be reserved to selected patients only.
Venous US should be proposed also in case of confirmed PE, for initial reference venous imaging, useful in case of DVT recurrence suspicion or further stratification in selected patients.
Venous US may be considered for further stratification in selected patients with concomitant suspected PE
Initial (first 5–21 days) and long-term (first 3–6 months) phase management
Deep vein thrombosis without pulmonary embolism
Anticoagulation in non-cancer patients
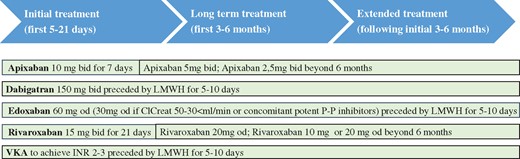
Deep vein thrombosis treatment consists of three phases (Figure3).39,Initial treatment (5–21 days following diagnosis); during this period, patients receive either parenteral therapy and are transited to vitamin K antagonists (VKA) or use high-dose direct oral anticoagulants (DOACs). Long-term treatment (following 3–6 months); patients are treated with VKA or DOACs.39 Initial and long-term treatments are mandatory for all DVT patients. Decision of extended treatment (beyond first 3–6 months) is based on benefit/risk balance of continued anticoagulation.
Deep vein thrombosis treatment phases. ClCreat: creatinine clearance; LMWH: low molecular weight heparin; P-P inhibitors: proton pump inhibitors; VKA: vitamin K antantagonist.
In patients with severe renal failure (creatinine clearance <30 mL/min), unstable renal function, or high bleeding risk, i.v. unfractionated heparin (UFH) may be preferable (short half-life and protamine sulfate reversibility). Less solid is the evidence in favor of UFH in obese (BMI >40 kg/m2), and underweight patients (<50 kg). Main disadvantage of UFH is its inter-individual dose variability requiring laboratory monitoring and dose adjustment. Additionally, UFH is associated with high risk of heparin-induced thrombocytopenia. For these reasons, low-molecular weight heparin (LMWH) is the parenteral treatment of choice. LMWHs are at least as effective as UFH and probably safer.40 Fondaparinux can also be used as parenteral agent.41 Both LMWH and fondaparinux do not have specific antidote.
Recently, DOACs have emerged as valid options for DVT treatment.39 Dabigatran and edoxaban were studied following initial 7–9 days treatment with a parenteral agent. Apixaban and rivaroxaban were evaluated by the ‘single drug approach’ (Figure3).
DOACs have longer elimination half-lives than UFH or LMWH and may accumulate in patients with suboptimal renal (creatinine clearance <30 mL/min) or hepatic function (Child-Pugh class B or C). Patients with poor renal and/or hepatic function, pregnancy/lactation, thrombocytopenia, were excluded from Phase III studies. Patients with active cancer were scarcely represented (3–8% of entire study population).
DOACs are at least as effective as and probably safer than parenteral drug/VKA treatment.42 A meta-analysis (27,023 patients) showed similar VTE recurrence rates in patients receiving DOACs or conventional therapy (2.0% vs 2.2%, RR 0.90). Major bleeding (RR 0.61), fatal bleeding (RR 0.36), intracranial bleeding (RR 0.37), and clinically relevant non-major bleeding (RR 0.73) were significantly lower in DOACs-treated patients.42 DOACs reversal agents are being investigated. Idarucizumab (Dabigatran reversal agent) is currently available for clinical use.43,44
Thrombolysis/thrombectomy
Early clot removal may prevent, at least partly, PTS developement.45
Catheter-directed thrombolysis (CDT) is more efficient than systemic lysis, mainly due to less bleeding, as thrombolytic agent is directly administered within the clot. Three major randomized controlled trials compared different CDT modalities on top of anticoagulation and compression, with a control group (anticoagulation and compression only). The CAVENT trial included 209 patients with first-time acute DVT (iliac, common femoral, and/or upper femoral vein).46 Adjuvant CDT was associated with a 26% RR PTS reduction over 2 years (41.1% vs. 55.6%, P = 0.04) compared with anticoagulation alone.46 Amount of residual post-CDT thrombus correlated with venous patency rates at 24-months (P = 0.04). Persistence of venous patency at 6 and 24 months correlated with PTS freedom (P < 0.001). A 3.2% of patients had major bleed, but there were no intracranial bleeds or deaths. Overall, trial found no differences in long-term (2 years) quality of life between patients with- or without CDT. Results have been confirmed after 5 years follow-up.47
Mechanical thrombus removal alone is not successful and needs adjuvant thrombolytic therapy. In PEARL I and II studies, only 5% of patients were treated without thrombolytics.48
Up to 83% of patients treated by any catheter-based therapy, need adjunctive angioplasty, and stenting.49 Primary acute DVT stenting is not recommended due to lack of data.
Vena cava filter
Vena cava filter may be used when anticoagulation is absolutely contraindicated in patients with newly diagnosed proximal DVT. One major complication is filter thrombosis. Therefore, anticoagulation should be started as soon as contraindications resolve50 and retrievable filter rapidly removed. Filter placement in addition to anticoagulation, does not improve survival51,52 except in patients with hemodynamically unstable PE or after thrombolytic therapy.53 Increased DVT recurrence has been shown with permanent51 but not with retrievable filters.52
Compression
Goal of compression is to relieve venous symptoms and eventually prevent PTS.54
Elastic compression stockings efficacy has been challenged by the SOX trial.55 A total of 806 patients with proximal DVT have been randomized to either 30–40 mmHg or placebo (<5 mmHg) stockings. Cumulative 2 years PTS incidence was similar (52.6% vs 52.3%; HR= 1.0). No difference in PTS severity or quality-of-life was observed.55 However, compliance definition (stockings wearing for ≥3 days/week) was significantly lower than in previous studies (56% vs ≈90%).56 Although role of stockings in PTS prevention may be uncertain, their use remains a reasonable option for controlling symptoms of acute proximal DVT.57
Compression associated with early mobilization and walking exercise has shown significant efficacy in venous symptom relieve in patients with acute DVT.58 Caution should be used in patients with severe peripheral artery disease.
Home vs in-hospital management
Most patients with DVT may be treated on a home basis (see Supplementary material online, only section).
Deep vein thrombosis with pulmonary embolism
Management of patients with acute PE is described in the 2014 ESC guideline6 (summary in the see Supplementary material online, only section).
Isolated distal deep vein thrombosis
Whether isolated distal DVT should be treated with anticoagulation is still debated. A recent trial randomized patients with a first isolated distal DVT to LMWH or placebo for 42 days.59 Rate of symptomatic proximal DVT or PE at 42 days was not different between LMWH and placebo (3.3% vs 5.4%); major or clinically relevant non-major bleeding occurred more frequently in the LMWH group (5 vs. 0, P = 0.03). These data seem to support that not all isolated distal DVT should receive full-dose anticoagulation.
Approach is to anticoagulate full-dose, for at least 3 months, as for proximal DVTs, patients at high-risk VTE (Table 3).60 Shorter LMWH treatment (4–6 weeks), even at lower doses, or ultrasound surveillance could be effective and safe in low-risk patients (Table 3).61 No data are available on DOACs. All patients with acute isolated distal DVT should be recommended to wear elastic stockings.62,63 Follow-up VUS is recommended to monitor thrombosis progression/evolution both in the presence or absence of anticoagulation.
Conditions or risk factors for complications after a first isolated distal DVT
| High-risk conditions . | Low-risk conditions . |
|---|---|
| Previous VTE events | Isolated distal DVT secondary to surgery or other transient risk factors (plasters, immobilization, trauma, long trip, etc.), provided complete mobilization is achieved |
| Males | Isolated distal DVT occurring during contraceptive or replacement hormonal therapy (provided therapy has been interrupted) |
| Age >50 years | |
| Cancer | |
| Unprovoked isolated distal DVT | |
| Secondary isolated distal DVT with persistently hampered mobilization | |
| Isolated distal DVT involving the popliteal trifurcation | |
| Isolated distal DVT involving >1 calf vein | |
| Isolated distal DVT present in both legs | |
| Presence of predisposing diseases (e.g. inflammatory bowel diseases) | |
| Known thrombophilic alterations | |
| Axial vs Muscular isolated distal DVT |
| High-risk conditions . | Low-risk conditions . |
|---|---|
| Previous VTE events | Isolated distal DVT secondary to surgery or other transient risk factors (plasters, immobilization, trauma, long trip, etc.), provided complete mobilization is achieved |
| Males | Isolated distal DVT occurring during contraceptive or replacement hormonal therapy (provided therapy has been interrupted) |
| Age >50 years | |
| Cancer | |
| Unprovoked isolated distal DVT | |
| Secondary isolated distal DVT with persistently hampered mobilization | |
| Isolated distal DVT involving the popliteal trifurcation | |
| Isolated distal DVT involving >1 calf vein | |
| Isolated distal DVT present in both legs | |
| Presence of predisposing diseases (e.g. inflammatory bowel diseases) | |
| Known thrombophilic alterations | |
| Axial vs Muscular isolated distal DVT |
Conditions or risk factors for complications after a first isolated distal DVT
| High-risk conditions . | Low-risk conditions . |
|---|---|
| Previous VTE events | Isolated distal DVT secondary to surgery or other transient risk factors (plasters, immobilization, trauma, long trip, etc.), provided complete mobilization is achieved |
| Males | Isolated distal DVT occurring during contraceptive or replacement hormonal therapy (provided therapy has been interrupted) |
| Age >50 years | |
| Cancer | |
| Unprovoked isolated distal DVT | |
| Secondary isolated distal DVT with persistently hampered mobilization | |
| Isolated distal DVT involving the popliteal trifurcation | |
| Isolated distal DVT involving >1 calf vein | |
| Isolated distal DVT present in both legs | |
| Presence of predisposing diseases (e.g. inflammatory bowel diseases) | |
| Known thrombophilic alterations | |
| Axial vs Muscular isolated distal DVT |
| High-risk conditions . | Low-risk conditions . |
|---|---|
| Previous VTE events | Isolated distal DVT secondary to surgery or other transient risk factors (plasters, immobilization, trauma, long trip, etc.), provided complete mobilization is achieved |
| Males | Isolated distal DVT occurring during contraceptive or replacement hormonal therapy (provided therapy has been interrupted) |
| Age >50 years | |
| Cancer | |
| Unprovoked isolated distal DVT | |
| Secondary isolated distal DVT with persistently hampered mobilization | |
| Isolated distal DVT involving the popliteal trifurcation | |
| Isolated distal DVT involving >1 calf vein | |
| Isolated distal DVT present in both legs | |
| Presence of predisposing diseases (e.g. inflammatory bowel diseases) | |
| Known thrombophilic alterations | |
| Axial vs Muscular isolated distal DVT |
Incidence of recurrent VTE appears to be similar to that of patients with proximal DVT.64,65
Consensus statement: initial and long-term management:
Patients with proximal DVT should be anticoagulated for at least 3-months.
Patients with isolated distal DVT at high-risk of recurrence should be anticoagulated, as for proximal DVT; for those at low risk of recurrence shorter treatment (4–6 weeks), even at lower anticoagulant doses, or ultrasound surveillance may be considered.
In the absence of contraindications, DOACs should be preferred as first-line anticoagulant therapy in non-cancer patients with proximal DVT.
Adjuvant CDT may be considered in selected patients with ilio-common femoral DVT, symptoms <14 days, and life expectancy >1 year if performed in experienced centres.
Primary acute DVT stenting or mechanical thrombus removal alone are not recommended.
Vena cava filters may be considered if anticoagulation is contraindicated, their use in addition to anticoagulation is not recommended.
Compression therapy associated with early mobilization and walking exercise should be considered to relieve acute venous symptoms.
Extended phase management (beyond first 3–6 months)
Duration of anticoagulation
Once anticoagulation is stopped, risk of VTE recurrence over years after a first episode is consistently around 30%.66 Risk is more than doubled in patients with unprovoked (annual rate >7.0%) vs those with (transient) provoked VTE,67 and among the latter in medical rather than surgical patients.68 Patients with a first symptomatic unprovoked DVT are at higher risk of recurrence than those with a first unprovoked PE.69 Factors related to DVT recurrence are listed in Table4.
For proximal DVT and/or PE, 3-months anticoagulation is the best option if transient and reversible risk factors were present.70 In all other patients, prolonging anticoagulation protects from recurrence (70–90%), but exposes to risk of unpredictable bleeding complications. Decision to discontinue or not anticoagulation should therefore be individually tailored and balanced against bleeding risk, taking also into account patients’ preferences. Three clinical prediction rules have been derived and prospectively validated to detect low-recurrence risk patients (Table4).71 A number of bleeding scores were evaluated, none showed sufficient predictive accuracy or had sufficient validation to be recommended in routine clinical practice.72,73
Continuing indefinite anticoagulation with the same drug administered during the first months is the best option for patients with multiple VTE episodes or strong VTE familial history, those with major thrombophilia, or longstanding medical diseases at high thrombotic risk.70 Indefinite anticoagulation can also be considered in patients with first episode of unprovoked VTE, especially in those with severe presentation, provided they are at low bleeding risk.70 Finally, discontinuing anticoagulation in non-cancer patients with repeatedly negative d-dimer (before drug interruption, 15, 30, 60, and 90 days following interruption) has proved to be safe in patients with unprovoked proximal DVT provided veins are recanalized or remained stable for 1 year.74 However, using moderately sensitive d-dimer assay during and 30 days after stopping anticoagulation, these results were not confirmed in men, and in women with VTE not associated with oestrogen treatment.75 Similarly, when measurements were repeated using a quantitative assay, d-dimer testing failed to identify subgroups with very low recurrence rate.76
Risk of recurrence after a first episode of unprovoked VTE
| Risk factors for DVT recurrence . | |||
|---|---|---|---|
| Proximal DVT location | Male sex | Persistence of residual vein thrombosis at ultrasound | |
| Obesity | Non-zero blood group | High d-dimer values | |
| Old age | Early PTS development | Role of inherited thrombophilia is controversial | |
| Clinical prediction rules assessing risk of recurrent VTE after first episode of unprovoked VTE71 | |||
| Score | Vienna prediction model | DASH score | HERDOO-2 |
| Parameters |
|
|
|
| Validation study | Yes | Yes | Yes |
| Commentaries | Different nomograms are available to calculate risk of VTE recurrence at different time | Patients with low score (≤1) have an annual recurrence rate of 3.1% | It is applicable in women only. Women with low score (≤1) have an annual recurrence rate of 1.3% |
| Risk factors for DVT recurrence . | |||
|---|---|---|---|
| Proximal DVT location | Male sex | Persistence of residual vein thrombosis at ultrasound | |
| Obesity | Non-zero blood group | High d-dimer values | |
| Old age | Early PTS development | Role of inherited thrombophilia is controversial | |
| Clinical prediction rules assessing risk of recurrent VTE after first episode of unprovoked VTE71 | |||
| Score | Vienna prediction model | DASH score | HERDOO-2 |
| Parameters |
|
|
|
| Validation study | Yes | Yes | Yes |
| Commentaries | Different nomograms are available to calculate risk of VTE recurrence at different time | Patients with low score (≤1) have an annual recurrence rate of 3.1% | It is applicable in women only. Women with low score (≤1) have an annual recurrence rate of 1.3% |
Risk of recurrence after a first episode of unprovoked VTE
| Risk factors for DVT recurrence . | |||
|---|---|---|---|
| Proximal DVT location | Male sex | Persistence of residual vein thrombosis at ultrasound | |
| Obesity | Non-zero blood group | High d-dimer values | |
| Old age | Early PTS development | Role of inherited thrombophilia is controversial | |
| Clinical prediction rules assessing risk of recurrent VTE after first episode of unprovoked VTE71 | |||
| Score | Vienna prediction model | DASH score | HERDOO-2 |
| Parameters |
|
|
|
| Validation study | Yes | Yes | Yes |
| Commentaries | Different nomograms are available to calculate risk of VTE recurrence at different time | Patients with low score (≤1) have an annual recurrence rate of 3.1% | It is applicable in women only. Women with low score (≤1) have an annual recurrence rate of 1.3% |
| Risk factors for DVT recurrence . | |||
|---|---|---|---|
| Proximal DVT location | Male sex | Persistence of residual vein thrombosis at ultrasound | |
| Obesity | Non-zero blood group | High d-dimer values | |
| Old age | Early PTS development | Role of inherited thrombophilia is controversial | |
| Clinical prediction rules assessing risk of recurrent VTE after first episode of unprovoked VTE71 | |||
| Score | Vienna prediction model | DASH score | HERDOO-2 |
| Parameters |
|
|
|
| Validation study | Yes | Yes | Yes |
| Commentaries | Different nomograms are available to calculate risk of VTE recurrence at different time | Patients with low score (≤1) have an annual recurrence rate of 3.1% | It is applicable in women only. Women with low score (≤1) have an annual recurrence rate of 1.3% |
Antithrombotics
Vitamin K antagonists
Four randomized studies evaluated VKA [target international normalized ratio (INR) 2.0–3.0] for VTE extended treatment in patients completing 3-months anticoagulation.77–80 Recurrent VTE occurred less in the VKA groups (combined OR 0.07).81 Bleeding was significantly higher.81
The ELATE study82 randomized patients to conventional intensity (INR 2.0–3.0), or low-intensity (INR 1.5–1–9). Recurrence rate was 0.7 vs 1.9/100 patient-years, respectively (HR 2.8), with no difference in major bleeding. Yet the low-intensity VKA therapy should be discouraged.
Direct oral anticoagulants
Dabigatran (150 mg b.i.d.) was as effective as warfarin and more effective than placebo in preventing recurrent VTE (Table5). Risk of major bleeding was reduced compared with warfarin.83
With Rivaroxaban (20 mg o.d.), risk of VTE recurrence was lower compared with placebo (HR 0.19), while bleeding risk was not increased (Table5).84 Standard and lower dose (10 mg od) also significantly reduced risk of recurrence compared to aspirine, without significant increase in bleeding.85
VTE recurrence occurred significantly less in standard and lower dose Apixaban (5 and 2.5 mg b.i.d.) vs placebo (Table5). Bleeding did not differ between groups.86
Recurrence rates with Edoxaban 60 mg were similar to the warfarin-treated group (post hoc analysis) (Table5).87 Major bleeding was lower in the edoxaban group.
Data from Phase IV studies are scarce, but results from XALIA are consistent with observations of rivaroxaban and warfarin.88
Extended secondary prevention of VTE: comparison of results from Phase III trials with direct oral anticoagulants
| Direct oral anticoagulant/trial . | Design . | n . | Treatment regimen . | Recurrent VTE or VTE-related death (% of population, HR) . | Major bleeding (% of population, HR) . | Intracranial hemorrhage (% of population, HR) . | Gastrointestinal bleeding (% of population, HR) . | Death from any cause (% of population, HR) . |
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
| |
| Randomized, double-blind | 1197 | Rivaroxaban 20 mg od vs. placebo |
|
| No intracranial hemorrhages observed |
|
|
| Randomized, double-blind | 2486 | Apixaban 5 mg b.i.d. or apixaban 2.5 mg b.i.d. vs. placebo |
|
|
|
|
|
| Randomized, double blind | 7227 | Edoxaban 60 mg qd (or dose reduced 30 mg) vs. warfarin |
|
|
| Not assessed | Not assessed |
| Data from comparative Phase IV studies | ||||||||
| Prospective, non-interventional | 5142 | Rivaroxaban 15 mg b.i.d. for 3 weeks followed by 20 mg qd vs. heparin/vitamin K antagonist |
|
| Not assessed | Not assessed |
|
| Direct oral anticoagulant/trial . | Design . | n . | Treatment regimen . | Recurrent VTE or VTE-related death (% of population, HR) . | Major bleeding (% of population, HR) . | Intracranial hemorrhage (% of population, HR) . | Gastrointestinal bleeding (% of population, HR) . | Death from any cause (% of population, HR) . |
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
| |
| Randomized, double-blind | 1197 | Rivaroxaban 20 mg od vs. placebo |
|
| No intracranial hemorrhages observed |
|
|
| Randomized, double-blind | 2486 | Apixaban 5 mg b.i.d. or apixaban 2.5 mg b.i.d. vs. placebo |
|
|
|
|
|
| Randomized, double blind | 7227 | Edoxaban 60 mg qd (or dose reduced 30 mg) vs. warfarin |
|
|
| Not assessed | Not assessed |
| Data from comparative Phase IV studies | ||||||||
| Prospective, non-interventional | 5142 | Rivaroxaban 15 mg b.i.d. for 3 weeks followed by 20 mg qd vs. heparin/vitamin K antagonist |
|
| Not assessed | Not assessed |
|
Extended secondary prevention of VTE: comparison of results from Phase III trials with direct oral anticoagulants
| Direct oral anticoagulant/trial . | Design . | n . | Treatment regimen . | Recurrent VTE or VTE-related death (% of population, HR) . | Major bleeding (% of population, HR) . | Intracranial hemorrhage (% of population, HR) . | Gastrointestinal bleeding (% of population, HR) . | Death from any cause (% of population, HR) . |
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
| |
| Randomized, double-blind | 1197 | Rivaroxaban 20 mg od vs. placebo |
|
| No intracranial hemorrhages observed |
|
|
| Randomized, double-blind | 2486 | Apixaban 5 mg b.i.d. or apixaban 2.5 mg b.i.d. vs. placebo |
|
|
|
|
|
| Randomized, double blind | 7227 | Edoxaban 60 mg qd (or dose reduced 30 mg) vs. warfarin |
|
|
| Not assessed | Not assessed |
| Data from comparative Phase IV studies | ||||||||
| Prospective, non-interventional | 5142 | Rivaroxaban 15 mg b.i.d. for 3 weeks followed by 20 mg qd vs. heparin/vitamin K antagonist |
|
| Not assessed | Not assessed |
|
| Direct oral anticoagulant/trial . | Design . | n . | Treatment regimen . | Recurrent VTE or VTE-related death (% of population, HR) . | Major bleeding (% of population, HR) . | Intracranial hemorrhage (% of population, HR) . | Gastrointestinal bleeding (% of population, HR) . | Death from any cause (% of population, HR) . |
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
| |
| Randomized, double-blind | 1197 | Rivaroxaban 20 mg od vs. placebo |
|
| No intracranial hemorrhages observed |
|
|
| Randomized, double-blind | 2486 | Apixaban 5 mg b.i.d. or apixaban 2.5 mg b.i.d. vs. placebo |
|
|
|
|
|
| Randomized, double blind | 7227 | Edoxaban 60 mg qd (or dose reduced 30 mg) vs. warfarin |
|
|
| Not assessed | Not assessed |
| Data from comparative Phase IV studies | ||||||||
| Prospective, non-interventional | 5142 | Rivaroxaban 15 mg b.i.d. for 3 weeks followed by 20 mg qd vs. heparin/vitamin K antagonist |
|
| Not assessed | Not assessed |
|
Aspirin
Two studies investigated aspirin 100 mg vs placebo in patients with idiopathic VTE who completed initial anticoagulation treatment.89,90 Pooled HR for VTE recurrence was 0.68 and 1.47 for bleeding.91
Other
Recent evaluation of Sulodexide vs placebo in patients with unprovoked VTE, who completed standard course of anticoagulation, showed a HR for VTE recurrence of 0.49 (P = 0.02).92 No major bleeding episodes were observed.
Venous occlusion recanalization
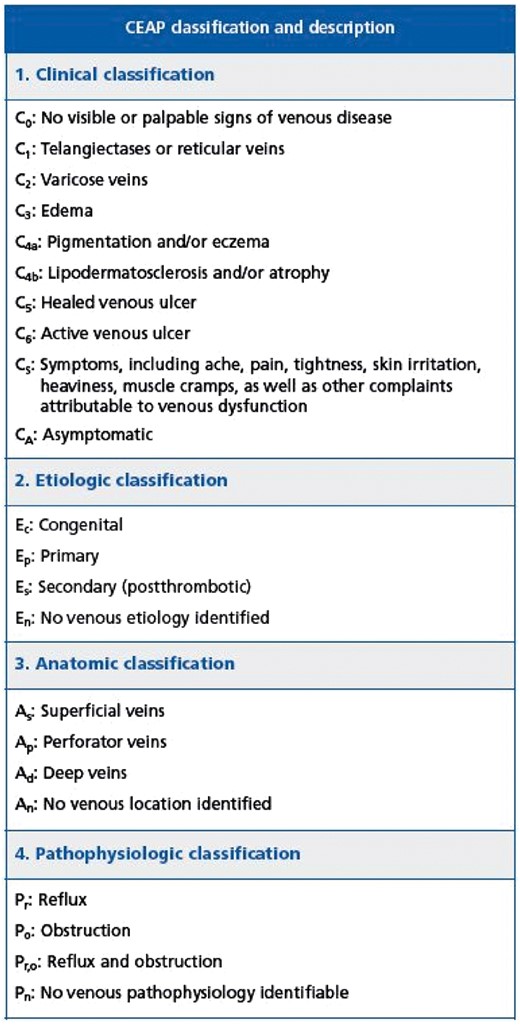
Endovascular techniques are available for selected patients with PTS.57 Case series and prospective cohort trials suggest that at least some subgroups of PTS patients (CEAP classes 4–6; Figure4) may benefit from addition of endovascular therapy into overall management strategy.
In patients with moderate-to-severe PTS and iliac vein obstruction, endovascular stent placement may be used to restore vein patency. In preliminary studies, stent placement in chronically occluded iliac veins contributed to ulcers healing, PTS symptoms relief, and reduced obstructive venous sequel.93
No randomized controlled trials are available, the largest series found patients with moderate-to-severe PTS to have reduced pain (P < 0.0001), severe pain (from 41% to 11%), and severe swelling (from 36% to 18%); increased ulcer healing (68%), and reduced venous pressure following recanalization with stent placement.93 Claudication improvement, better outflow fraction, and calf pump function was also observed.94
In selected infrequent cases, surgical vein bypass may be an option to relieve venous hypertension.
Follow-up
Patients with DVT should be followed to avoid risk of recurrence as well as DVT and anticoagulation-related complications. Development of renal failure, changes in body weight, or pregnancy that may require anticoagulation adjustment should be monitored. Compliance as well as benefit/risk balance should be assessed regularly. VUS, at anticoagulation discontinuation, is useful in determining baseline residual vein thrombosis.
Consensus statement: extended management:
Decision to discontinue or not anticoagulation should be individually tailored, balancing risk of recurrence against bleeding risk, taking into account patients’ preferences and compliance.
In the absence of contraindications, DOACs should be preferred as first line anticoagulant therapy in non-cancer patients. Currently low-dose apixaban and rivaroxaban have shown their benefit in this setting.
When VKAs are proposed, they should be administered at conventional intensity regimen (INR 2–3).
Aspirin may be considered for extended treatment if anticoagulation is contraindicated.
Endovascular recanalization may be considered in patients with chronic venous occlusion class CEAP 4–6.
Regular (at least yearly) assessment of compliance and benefit/risk balance should be performed in patients on extended treatment.
At anticoagulation discontinuation, venous US should be performed to establish a baseline comparative exam in case of recurrence.
Special situations
Upper extremities deep vein thrombosis
Upper extremities DVT (UEDVT) accounts for 10% of all DVTs with an annual incidence of 0.4–1.0/10.000 persons.95,96 Incidence rises because of increasing use of central venous catheters, cardiac pacemakers, and defibrillators.95,96 Complications are similar, although less frequent, to those of lower limb DVT.95,96 About 20–30% of UEDVT are primary comprising those caused by anatomic abnormalities or following sustained physical efforts.97 Secondary DVT include venous catheter- and devices-related complications, cancer, pregnancy, and recent arm/shoulder surgery or trauma. Most common clinical presentation includes pain, swelling, and skin discoloration. A clinical decision score has been proposed (Table6).98,d-Dimer showed good negative predictive value in symptomatic DVT.99,100 VUS is the first choice exam for diagnosis.101
A diagnostic algorithm, using Constans score, d-dimer, and VUS was proposed (Table6).100 Contrast-, CT-, and MR-venography are not recommended for diagnosis but limited to unresolved selected cases.96 Anticoagulation is similar to that of lower limb DVT. Thrombolysis is not routinely recommended but limited to selected severe cases. A prognostic score identifying low-risk DVT patients who could be safely treated at home has been proposed but not yet externally validated.102
Constans clinical score for UEAD
| Constans score: item . | Risk score . |
|---|---|
| Central venous catheter or pacemaker thread | 1 |
| Localized pain | 1 |
| Unilateral edema | 1 |
| Other diagnosis at least as plausible | −1 |
| Constans score: item . | Risk score . |
|---|---|
| Central venous catheter or pacemaker thread | 1 |
| Localized pain | 1 |
| Unilateral edema | 1 |
| Other diagnosis at least as plausible | −1 |
Score ≤1 = Upper extremity DVT unlikely.
Score ≥2 = Upper extremity DVT likely.
Constans clinical score for UEAD
| Constans score: item . | Risk score . |
|---|---|
| Central venous catheter or pacemaker thread | 1 |
| Localized pain | 1 |
| Unilateral edema | 1 |
| Other diagnosis at least as plausible | −1 |
| Constans score: item . | Risk score . |
|---|---|
| Central venous catheter or pacemaker thread | 1 |
| Localized pain | 1 |
| Unilateral edema | 1 |
| Other diagnosis at least as plausible | −1 |
Score ≤1 = Upper extremity DVT unlikely.
Score ≥2 = Upper extremity DVT likely.
Deep vein thrombosis at unusual sites
Cerebral vein thrombosis
Most common cerebral vein thrombosis (CVT) presentation includes severe headaches, seizures, focal neurological deficits, and altered consciousness.103,104 For the diagnosis and treatment refer to the Supplementary material online, only section.
Splanchnic vein thrombosis
Splanchnic vein thrombosis may present as sudden onset of abdominal pain with or without other non-specific abdominal symptoms.105,106 Upper gastrointestinal bleeding or abrupt ascites worsening may occur in cirrhotic patients, lower gastrointestinal bleeding, or acute abdomen may occur in patients with mesenteric vein thrombosis.105 For the diagnosis and treatment refer to the Supplementary material online, only section.
Deep vein thrombosis and cancer
Cancer patients show four- to seven-fold increased VTE risk (second cause of death). Incidental VTE is increasingly diagnosed and associated with worse overall survival. VTE risk varies from cancer diagnosis through treatment, with annual incidence rate of 0.5–20% according to cancer site and type, metastasis status, treatment (surgery, chemotherapy), use of central venous catheters, hospitalization, and patient-related factor. Risk-assessment models may help stratify individual VTE risk and tailor adequate therapy (Table7).107–109
Khorana decision score in cancer patients
| Khorana score: patient characteristic . | Risk score . |
|---|---|
| Site of cancer | |
| Very high risk (stomach, pancreas) | 2 |
| High risk (lung, lymphoma, gynecologic, bladder, testicular) | 1 |
| Pre-chemotherapy platelet count 350 × 109/L or more | 1 |
| Hemoglobin level <10 g/dL or use of red cell growth factors | 1 |
| Pre-chemotherapy leukocyte count >11 × 109/L | 1 |
| BMI ≥35 kg/m2 | 1 |
| Khorana score: patient characteristic . | Risk score . |
|---|---|
| Site of cancer | |
| Very high risk (stomach, pancreas) | 2 |
| High risk (lung, lymphoma, gynecologic, bladder, testicular) | 1 |
| Pre-chemotherapy platelet count 350 × 109/L or more | 1 |
| Hemoglobin level <10 g/dL or use of red cell growth factors | 1 |
| Pre-chemotherapy leukocyte count >11 × 109/L | 1 |
| BMI ≥35 kg/m2 | 1 |
Score ≥3 = high risk; Score 1−2= intermediate risk; Score 0 = low risk.
Khorana decision score in cancer patients
| Khorana score: patient characteristic . | Risk score . |
|---|---|
| Site of cancer | |
| Very high risk (stomach, pancreas) | 2 |
| High risk (lung, lymphoma, gynecologic, bladder, testicular) | 1 |
| Pre-chemotherapy platelet count 350 × 109/L or more | 1 |
| Hemoglobin level <10 g/dL or use of red cell growth factors | 1 |
| Pre-chemotherapy leukocyte count >11 × 109/L | 1 |
| BMI ≥35 kg/m2 | 1 |
| Khorana score: patient characteristic . | Risk score . |
|---|---|
| Site of cancer | |
| Very high risk (stomach, pancreas) | 2 |
| High risk (lung, lymphoma, gynecologic, bladder, testicular) | 1 |
| Pre-chemotherapy platelet count 350 × 109/L or more | 1 |
| Hemoglobin level <10 g/dL or use of red cell growth factors | 1 |
| Pre-chemotherapy leukocyte count >11 × 109/L | 1 |
| BMI ≥35 kg/m2 | 1 |
Score ≥3 = high risk; Score 1−2= intermediate risk; Score 0 = low risk.
Cancer-related VTE is at high risk of recurrence and bleeding during treatment, risk of death increases up to eight-fold following acute VTE compared with non-cancer patients. LMWH is recommended for initial treatment (similar efficacy and higher safety than UFH). Fondaparinux in patients with history of heparin-induced thrombocytopenia, and UFH in case of renal failure are valid alternatives. Vena cava filter and thrombolysis should only be considered on a case-by-case basis. For long-term treatment, superiority of LMWH over short-term heparin followed by VKA is well documented. LMWH used during at least 3 and up to 6 months when compared with VKA significantly reduced VTE recurrence with similar safety profile. After 6 months, termination or continuation of anticoagulation should be individually evaluated: benefit–risk ratio, tolerability, patients’ preference, and cancer activity.110
In symptomatic catheter-related thrombosis, anticoagulation is recommended for at least 3-months. LMWHs are suggested although VKAs can also be used (no direct comparison available). Central-vein-catheter can be maintained in place if it is functional, non-infected, and there is good thrombosis resolution. Optimal anticoagulation duration has not been determined, however, 3-months duration seems acceptable in analogy with upper extremity DVT (UEDVT).110
For VTE recurrence under proper anticoagulation (INR, antiXa within therapeutical range), 3 options are recommended: (i) switch from VKA to LMWH in patients treated with VKA; (ii) increase weight-adjusted dose of LMWH by 20–25%; (iii) vena cava filter use, although no specific results are available for cancer patients.
No direct comparison of DOACs with LMWH is currently available. Nevertheless, data from recent large VTE trials showed non-inferiority in terms of efficacy and safety of DOACs compared with AVK in cancer patients included in the studies.111
Deep vein thrombosis in pregnancy
VTE remains the leading cause of maternal mortality in industrialized world.112 VTE risk factors are listed in Table8. Validity of DVT clinical prediction rules in pregnancy has not yet been tested prospectively.113 The LEFt clinical score was proposed.113 Although d-dimers increase during pregnancy, normal values exclude VTE with likelihood similar to non-pregnant women.6 VUS is the primary imaging test.114,115 Unless contraindicated, anticoagulation should be initiated until objective testing.115,116 If VUS is negative but clinical suspicion high, testing should be repeated.117,118 Rarely, CT or MRI venography may be considered.
VTE risk factors during pregnancy
| Prior VTE | Preterm delivery |
| Smoking | Pre-eclampsia |
| Varicosis | Caesarean section (specifically in the emergency situation) |
| Hyperemesis | Postpartum infection or hemorrhage |
| severe thrombophilia | Transfusion |
| assisted reproductive technology | Immobilization |
| BMI >30 kg/m² | Systemic lupus erythematosus |
| Prior VTE | Preterm delivery |
| Smoking | Pre-eclampsia |
| Varicosis | Caesarean section (specifically in the emergency situation) |
| Hyperemesis | Postpartum infection or hemorrhage |
| severe thrombophilia | Transfusion |
| assisted reproductive technology | Immobilization |
| BMI >30 kg/m² | Systemic lupus erythematosus |
VTE risk factors during pregnancy
| Prior VTE | Preterm delivery |
| Smoking | Pre-eclampsia |
| Varicosis | Caesarean section (specifically in the emergency situation) |
| Hyperemesis | Postpartum infection or hemorrhage |
| severe thrombophilia | Transfusion |
| assisted reproductive technology | Immobilization |
| BMI >30 kg/m² | Systemic lupus erythematosus |
| Prior VTE | Preterm delivery |
| Smoking | Pre-eclampsia |
| Varicosis | Caesarean section (specifically in the emergency situation) |
| Hyperemesis | Postpartum infection or hemorrhage |
| severe thrombophilia | Transfusion |
| assisted reproductive technology | Immobilization |
| BMI >30 kg/m² | Systemic lupus erythematosus |
Treatment is based on heparin anticoagulation (no placenta crossing and not significantly found in breast milk).6 LMWHs are safe in pregnancy,119–121 anti-Xa monitoring, and dose adaptation cannot be recommended routinely, but may be considered in women at extremes of body-weight or renal disease.6 Whether initial full dose anticoagulation can be reduced to intermediate dose for secondary prevention during ongoing pregnancy remains unclear.120 Dose reduction should be considered for women at high risk of bleeding, osteoporosis, or low VTE recurrence risk.116 Evidence is insufficient to recommend o.d. or b.i.d. LMWH, but b.i.d. may be more suitable perinatally to avoid high anti-Xa levels at time of delivery. Anticoagulation should be continued for at least 6 weeks postnatally and until at least a total of 3 months treatment.117
Consensus statement: DVT management in special situations:
In case of UEDVT suspicion, venous US is the first choice imaging test.
Treatment of UEDVT is similar to that of lower limb DVT with regard to anticoagulation.
LMWH are recommended for acute treatment of CVT.
LMWH are recommended for acute treatment of splanchnic vein thrombosis.
LMWH are recommended for initial and long-term treatment in cancer patients.
In cancer patients, after 6 months, decision of continuation and, if so, the mode of anticoagulation should be based on individual evaluation of the benefit-risk ratio, tolerability, patients’ preference, and cancer activity.
During pregnancy, venous US is recommended as first line DVT imaging test.
During pregnancy, LMWH is recommended for initial and long-term treatment.
Anticoagulant treatment should be continued for at least 6 weeks after delivery with a total of 3-months treatment.
Conflict of interest: Dr. Mazzolai reports personal fees from Bayer Health Care, personal fees from Pfizer - Bristol-Myers Squibb, personal fees from Daiichi-Sankyo, outside the submitted work. Dr. Aboyans reports personal fees from Bayer Healthcare, personal fees from Boehringer Ingelheim, personal fees from Daichii-Sankyo, personal fees from Astra-Zeneca, personal fees from Sanofi, personal fees from MSD, personal fees from BMS/Pfizer alliance, personal fees from Novartis, outside the submitted work. Dr. Ageno reports grants and personal fees from Bayer, grants and personal fees from Boehringer Ingelheim, personal fees from Daiichi Sankyo, personal fees from BMS-Pfizer, personal fees from Aspen, outside the submitted work. Dr. Agnelli reports personal fees from Bristol-Myers-Squibb, personal fees from Pfizer, personal fees from Bayer Healthcare, personal fees from Boehringer Ingelheim, personal fees from Daiichi Sankyo, outside the submitted work. Dr. Alatri reports personal fees from Bayer Health Care, personal fees from Pfizer-Bristol-Myers-Squibb, outside the submitted work. Dr Bauersachs reports personal fees from Bayer Healthcare, Boehringer Ingelheim, Pfizer - Bristol-Myers Squibb, Daichii-Sankyo, outside the submitted work. Dr. Buller reports grants from Sanofi-Aventis, personal fees from Sanofi-Aventis, grants from Bayer HealthCare, personal fees from Bayer HealthCare, grants from Bristol-Myers-Squibb, personal fees from Bristol-Meyers-Squibb, grants from Daiichi Sankyo, personal fees from Daiichi Sankyo, grants from Glaxo SmithKline, personal fees from Glaxo SmithKline, grants from Pfizer, personal fees from Pfizer, grants from Roche, personal fees from Roche, grants from Isis, personal fees from Isis, grants from Thrombogenics, personal fees from Thrombogenics, during the conduct of the study. Dr. ELIAS reports grants from Bayer Pharma, personal fees from Bayer Pharma, grants from Daiichi San§rma, personal fees from Daiichi San§o Pharma, outside the submitted work. Dr Farge reports other from Portola, non-financial support from Leo Pharma, non-financial support from Aspen, non-financial support from Pfizer, outside the submitted work. Dr. Konstantinides reports grants and personal fees from Bayer Health Care, grants and personal fees from Boehringer Ingelheim, grants and personal fees from Daiichi Sankyo, personal fees from Pfizer - Bristol-Myers Squibb, outside the submitted work. Dr. Palareti reports personal fees from Alfa-Wassermann, personal fees from Daiichi-Sankyo, personal fees from Siemens, personal fees from Werfen, outside the submitted work. Dr. Torbicki reports grants and personal fees from Bayer Healthcare, grants from Pfizer, outside the submitted work. Dr. Vlachopoulos reports personal fees from Bayer, reports personal fees from Merck Sharp &Dome, reports personal fees from Angelini, reports personal fees from Pfizer, reports personal fees from Astra Zeneca, reports personal fees from Menarini, reports personal fees from Elpen, reports personal fees from Merck reports personal fees from Serono, reports personal fees from Novartis, reports personal fees from Boehringer-Ingelheim, reports personal fees from OMRON, reports personal fees from Sanofi Aventis, reports personal fees from PharmaSuiss, reports personal fees from Amgen, outside of the submitted work.
Footnotes
The opinions expressed in this article are not necessarily those of the Editors of the European Heart Journal or of the European Society of Cardiology.